Abstract
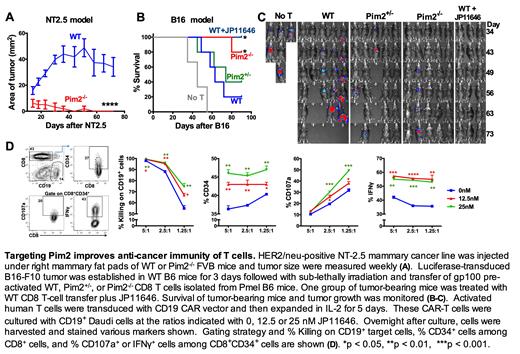
The Provirus Integration sites for Moloney murine leukemia virus (Pim) kinases are a highly conserved family of serine/threonine kinases. The Pim kinase family is composed of three different isoforms, Pim1, Pim2, and Pim3, which have been studied extensively in tumorigenesis and as a potential therapeutic target in various cancers. We previously reported an unexpected role of Pim2 in negatively regulates T-cell responses to alloantigen and tumor (JCI, 2015, PMID: 29781812). However, the mechanisms by which Pim2 modulates T-cell responses remain largely undefined. In the current study, using genetic Pim2-deficient mouse, we demonstrated a key role of Pim2 in regulating T-cell hemostatic and anti-tumor responses in aging, hematopoietic cell transplantation (HCT), and antigen-specific adoptive T-cell therapy (ACT). We observed that Pim2 was critical for T cells to retain quiescent in aged mice, as thymic Treg development was impaired while effector T-cell differentiation in lymphoid organs, including Tc1/Th1, Tc17/Th17 and follicular helper T cells, was increased in Pim2-deficient mice, but not in Pim1/Pim3-deficient mice. Furthermore, Pim2-deficient mice were capable to completely eradicate syngeneic breast cancer (NT2.5) growth (Figure A). During antigen specific anti-tumor response, adoptively transferred Pim2 -/- CD8 T cells showed enhanced ability for controlling established NT2.5 breast cancer and B16 melanoma (Figure B, C). Mechanistically, loss of Pim2 promoted G1 to S phase cell-cycle progression while reduced apoptosis in CD8 T cells. Pim2 -/- CD8 T cells exhibited elevated effector cytokine production while maintained higher levels of CD62L expression, leading to superior effector function, persistence and anti-tumor activity. Reduced differentiation of exhausted and suppressive subsets were observed in Pim2 -/- CD8 T cells after being adoptively transferred in tumor-bearing mice. In addition, Pim2 deficiency was associated with a higher metabolic potential, reflected by increased glycolysis and oxidative phosphorylation, which was at least partially attributed to a decreased level of autophagy in Pim2 -/- CD8 T cells. To further evaluate the clinical translation potential, we applied a Pim2-specific inhibitor (JP11646) and found that blocking Pim2 improved graft-versus-leukemia activity after autologous HCT and also enhanced CD8 T-cell mediated anti-melanoma effects after ACT in mice (Figure B, C). Furthermore, blocking Pim2 using JP11646 promoted human CD8 T-cell response during polyclonal stimulation and enhanced expansion, effector function and tumor killing ability of human melanoma antigen-specific CD8 T cells (data not shown) and CD19 CAR-T cells (Figure D). Our work demonstrated that Pim2 is a potent and distinct regulator of differentiation and maintenance of T effector cells through modulating metabolism and autophagy. Specifically target Pim2 can serve as a novel strategy for improving cancer immunotherapy.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal