Abstract
Introduction
Omidubicel is a hematopoietic stem cell graft derived from umbilical cord blood, composed of an ex vivo nicotinamide-expanded CD133+ stem cell fraction and a non-expanded CD133- T-cell-containing fraction. Omidubicel was the first ex vivo expanded stem cell graft to demonstrate durable engraftment and the first to be transplanted as a single, stand-alone graft following myeloablative conditioning. Recent prospective clinical trials have established the short-term safety and efficacy of hematopoietic cell transplantation (HCT) with omidubicel, both as a stand-alone graft and in conjunction with an unmanipulated cord blood unit (Horwitz et al, 2014, 2019, and 2021). This retrospective study reports on the long-term experience with omidubicel HCT in patients with advanced hematologic malignancies at a single institution.
Methods
All patients with hematologic malignancies who had undergone HCT and engrafted with omidubicel between November 2010 and January 2020 were eligible for analysis. Patients who experienced primary graft failure or engrafted with an unmanipulated unit were excluded. A retrospective review was performed under the auspices of an IRB approved protocol to determine their long-term outcomes. The primary endpoints were survival and long-term hematopoiesis. Secondary endpoints included immune reconstitution, disease relapse, chronic graft versus host disease (GVHD), and secondary hematologic malignancies. R 4.1.0 was used to perform Kaplan Meier survival analysis and competing risk analyses of cumulative incidence via Gray's method.
Results
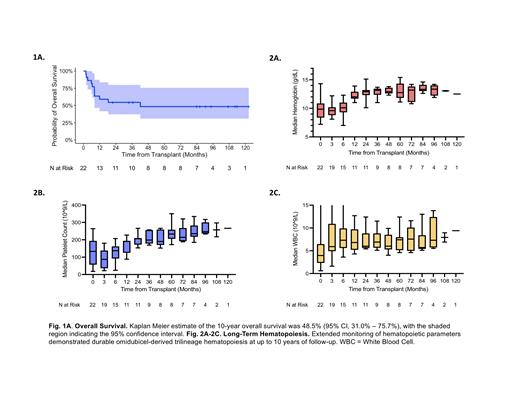
A total of 26 patients received an omidubicel graft during the study period, of whom 22 were eligible for analysis. Three patients engrafted with an unmanipulated cord blood graft and 1 patient experienced primary graft failure. The median age of the eligible patients was 48 yrs (range, 18 - 62 yrs) and 8 patients (36%) were non-white. The most common disease types were AML (36%), MDS (32%), and ALL (18%). By disease risk index, 5 patients (23%) were considered high risk, 13 (59%) were intermediate risk, 3 (14%) were low risk, and 1 (5%) was unevaluable. At the time of data cutoff, the median follow-up was 2.3 yrs (range, 0.1 - 10 yrs) for all eligible patients and 7.4 yrs (range, 1.8 - 10 yrs) for the 11 survivors.
At last follow-up, 11 patients had passed away due to disease relapse (64%), acute GVHD (27%), or infection (9%). The estimated 10-year overall survival and disease-free survival were 48.5% (95% CI, 31.0% - 75.7%) (Fig 1A) and 43.6% (95% CI, 26.8% - 70.9%), respectively. Durable omidubicel-derived trilineage hematopoiesis was observed at up to ten years of follow-up (Fig 2A-2C). Serial quantitative assessments of lymphocyte subsets suggest stability of the median CD3+, CD4+, and CD8+ T cell, as well as CD19+ B cell and NK cell counts. These levels were within normal ranges starting at 6 months post-transplant and up until 8 years of follow-up.
Using competing risk analysis, the 10-year estimated cumulative incidence of relapse was 31.8% (95% CI, 13.6% - 51.8%). The 10-year estimated cumulative incidence of chronic GVHD was 31.8% (95% CI, 13.4% - 52.1%). Of the 7 patients who experienced chronic GVHD, only 2 still required systemic immunosuppression at last follow-up. One patient experienced secondary graft failure requiring a rescue haploidentical transplant on day 202. There were no cases of secondary hematologic malignancies.
Conclusions
This study suggests that omidubicel provides long-term sustainable hematopoiesis and immune competence at follow-up periods of up to 10 years. This is the first characterization of the long-term safety of an ex vivo expanded hematopoietic stem cell graft. While there has been concern that ex vivo expansion approaches may compromise the integrity of long-term repopulating hematopoietic stem cells, there was only one documented case of secondary graft failure in this cohort. Furthermore, there was no evidence for the development of clonal hematopoiesis, however molecular testing was not performed to confirm this observation. Chronic GVHD was observed, but most patients were able to wean off immunosuppression. In summary, omidubicel is a safe and reliable stem cell source for patients in need of hematopoietic cell transplantation, particularly for underrepresented racial and ethnic minorities.
Choi: Janssen Biotech: Speakers Bureau. Gasparetto: Janssen: Consultancy; Sanofi: Consultancy, Honoraria, Speakers Bureau; Oncopeptide: Consultancy, Honoraria, Speakers Bureau; Gsk: Consultancy, Honoraria, Speakers Bureau; Karyopharm: Consultancy, Honoraria, Speakers Bureau. Lopez: PhosphoGam: Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company. Rizzieri: AbbVie: Membership on an entity's Board of Directors or advisory committees; AROG: Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Teva: Membership on an entity's Board of Directors or advisory committees; Acrotech: Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Stemline: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kite: Honoraria, Speakers Bureau; Karyopharm: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees; Celltrion: Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Mustang: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Jazz: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Honoraria; Pharmacyclics: Honoraria. Sarantopoulos: Rigel: Other: Advisory Board. Sung: Merck: Research Funding; Novartis: Research Funding; Enterome: Research Funding; Seres: Research Funding; AVROBIO: Consultancy; Abbott Nutrition: Honoraria; Clasado: Other: Research Product; DSM: Other: Research Product. Galamidi-Cohen: Gamida Cell, Ltd: Current Employment. Schwarzbach: Gamida Cell: Current Employment. Horwitz: Gamida Cell: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal