Abstract
Background:
Patients with end-stage renal disease (ESRD) have been excluded from pivotal clinical trials with novel oral anticoagulants (NOACs). These patients are both at increased risk of venous and arterial thromboembolism and the risk of bleeding is also elevated, thus making treating such patients a challenge. Patients with ESRD and atrial fibrillation (AF) additionally have an increased risk of stroke, compared with less severe renal impairment. The risk of venous thromboembolism (VTE) is also positively correlated with CKD, suggesting ESRD is a hypercoagulable stage.
Vitamin K antagonists (VKAs) such as warfarin are the mainstay of treatment as prevention or treatment of thromboembolic disease in ESRD, although the use is highly debated. Randomized controlled trials (RCTs) evaluating DOACs in AF all demonstrated non-inferiority for stroke or side effects, however these studies excluded patients on hemodialysis (HD) and those with creatinine clearance less than 25-30. There appears to be a dearth of high-quality evidence regarding use of DOACs in patients with ESRD. This retrospective analysis aims to evaluate whether use of NOACS causes increased bleeding rates compared to warfarin in the specific population of ESRD patients.
Methods:
Our study was a retrospective analysis of data using chart review of patients from an urban community medical center between the years 2017-2020. Retrospective data was entered into an electronic data form. Patients required a documented diagnosis of ESRD on hemodialysis and must have received at least one dose of anticoagulation to be included in analysis. Retrospective data pertaining to bleeding events were extracted for this analysis, including GI bleeding, intracranial bleeding, or any other major bleeding. Major bleeding events were defined as those requiring hospitalization or blood transfusion, or any other specific medical intervention. Demographics and clinical variables were presented using frequency and percentages. Patients were stratified into two groups, NOAC use and warfarin use. Continuous variables were analyzed using independent T test while chi square test was used for categorical variables. A p value <0.05 was considered statistically significant.
Results:
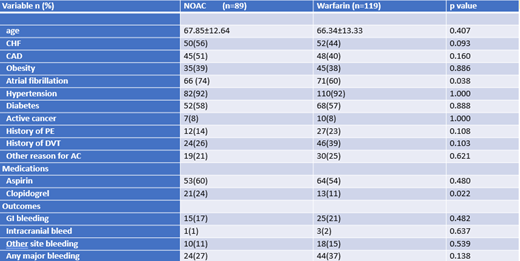
A total sample of 208 patients with ESRD on dialysis who had received anticoagulation were included in this analysis. 51% of patients were male and the majority were African American (79%). The mean age was 67. 43% of patients were on NOACs while 57% were on warfarin. Several other clinical factors were also recorded, with 38% of patients being obese, 92% with hypertension, 58% with diabetes, 45% with coronary artery disease, and 67% with atrial fibrillation. Bleeding outcomes were recorded for both patients who had received a NOAC or warfarin. 24 bleeding events were noted in NOAC group versus 44 events in the warfarin group. There was a higher rate of any major bleeding in the warfarin group versus NOAC group (37% vs 27%), however this not statistically significant (p=0.138)
Limitations:
More data will need to be collected to gain statistical significance of primary endpoint findings, and we have estimated that a total of approximately 680 patient outcomes would need to be analyzed to gain statistical significance. This data collection is currently underway. Other limitations include the retrospective nature of this study, with heavy reliance on review of patient charts for proper documentation of clinically relevant bleeding while on anticoagulation. Ideally, a prospective study would be better able to standardize data.
Conclusions:
Based on current data analysis, there is a trend towards warfarin having higher rates of bleeding compared to DOACs in ESRD patients on dialysis, 37% versus 27%, although this is only approaching statistical significance. These findings may shift current lines of thought that warfarin is a more acceptable choice of anticoagulation in ESRD patients given long term experience with this drug. Further studies are needed to make more definite conclusions.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal