Abstract
Background: Patients with B-cell lymphoma have poor clinical outcomes to SARS-CoV-2 infection (COVID-19) and are also more likely to have suboptimal responses to immunization. An understanding of which lymphoma patients are at greatest risk of poor COVID-19 vaccine response and what aspects of immunity are most impaired is critical for developing strategies to protect these patients from a potentially fatal infection.
Methods: We enrolled 149 participants, including 129 with lymphoma and 20 age-matched controls, who received a complete COVID-19 vaccination series to assess how B- and T-cell vaccine responses vary among clinically relevant subgroups and over time. This cohort included 99 patients with prior anti-CD20 treatment, ranging from 1 week to 17 years prior, allowing us to assess relationships between timing and intensity of anti-CD20 exposure and vaccine response. The cohort also included 18 patients who began treatment with an anti-CD20-containing regimen after being fully vaccinated, in whom we are assessing the potential efficacy of a pre-therapy vaccination strategy. Peripheral blood samples were taken on average 28 days and 4 months after last vaccine dose. B-cell responses are being profiled by measuring anti-Spike serum IgG, RBD-ACE2 blocking activity, and spike-specific memory B cells. T cell assessments include quantitation of spike-specific activation and cytokine production via interferon-gamma ELISPOTs and multiparameter flow cytometry.
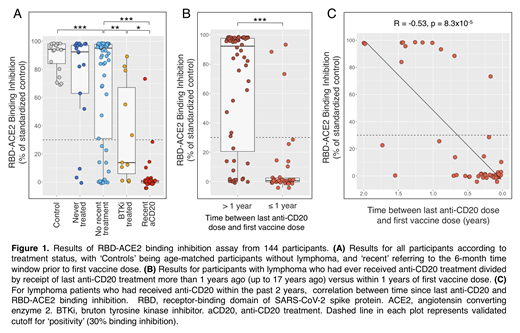
Results: The 129 participants with lymphoma had a median age of 68, were 59% male, and 70% had either diffuse large B-cell lymphoma (DLBCL) or follicular lymphoma (FL). Thirteen percent had no therapy prior to vaccination, half had not been treated in the past six months and 36% had been treated recently or were currently being treated. Ninety-three percent received an mRNA COVID-19 vaccine. Although we did not detect a statistically significant difference in vaccine response between previously untreated lymphoma patients and controls, 3/17 were negative for blocking antibodies post-vaccine, compared to 0/20 controls (Fishers exact test p=0.09, Figure 1A). Lymphoma patients with any history of treatment had impaired serologic responses to the vaccine compared to previously untreated patients (p=0.01). Responses did not vary by specific lymphoma histology. Blocking antibodies were evident in only 45% and 3% of patients recently/currently treated with a BTK inhibitor or an anti-CD20 antibody, respectively (Figure 1A). Closely evaluating all patients who had ever received anti-CD20 antibody therapy, we found a strong linear correlation between time since last anti-CD20 treatment and RBD-ACE2 binding inhibition (p<0.0001, Figure 1B-C). T cell and memory B cell assays are ongoing as is analysis of response persistence and of the pre-therapy vaccination cohort, and results from these efforts will be included in the final presentation.
Conclusions: Treatment with anti-CD20 antibodies significantly impaired COVID-19 vaccine-induced humoral responses in patients with lymphoma in a manner dependent on the time elapsed since last anti-CD20 treatment. Vaccination at least six months after anti-CD20 treatment, likely co-incident with recovery of the B-cell compartment, was associated with positive blocking antibody titers. These data suggest that booster vaccination strategies are more likely to succeed in lymphoma patients who have not received anti-CD20 treatment for at least 6 months and that those patients with recent anti-CD20 treatment may benefit most from passive immunization strategies. Forthcoming results from the pre-therapy vaccination cohort will also help inform sequencing of additional vaccine doses, passive immunization, and anti-cancer treatments.
Shree: Gilead: Other: Spouse's employment. Beygi: Kite/Gilead: Current Employment. Advani: Astellas/Agensys: Research Funding; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Bayer: Membership on an entity's Board of Directors or advisory committees; Bristol Myer Squibb: Membership on an entity's Board of Directors or advisory committees; Cell Medica: Membership on an entity's Board of Directors or advisory committees; Forty Seven: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genetech Inc.: Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Membership on an entity's Board of Directors or advisory committees; Janssen Pharmaceutical: Research Funding; Juno: Membership on an entity's Board of Directors or advisory committees; Kite Pharma: Membership on an entity's Board of Directors or advisory committees; Kura: Research Funding; Kyowa: Membership on an entity's Board of Directors or advisory committees; Merck: Research Funding; Millenium: Research Funding; Pharmacyclics: Consultancy, Research Funding; Portola Pharmaceuticals: Consultancy; Regeneron: Research Funding; Roche: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Seattle Genetics: Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees. Khodadoust: CRISPR Therapeutics, Nutcracker Therapeutics: Research Funding; Myeloid Therapeutics: Membership on an entity's Board of Directors or advisory committees; Alexion, AstraZeneca Rare Disease: Other: Study investigator. Kurtz: Roche: Consultancy; Foresight Diagnostics: Consultancy, Current holder of stock options in a privately-held company; Genentech: Consultancy. Alizadeh: Bristol Myers Squibb: Research Funding; Gilead: Consultancy; Celgene: Consultancy, Research Funding; Janssen Oncology: Honoraria; Roche: Consultancy, Honoraria; Foresight Diagnostics: Consultancy, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company; Forty Seven: Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company; CAPP Medical: Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company; Cibermed: Consultancy, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company. Levy: GigaGen: Membership on an entity's Board of Directors or advisory committees; Teneobio: Membership on an entity's Board of Directors or advisory committees; Nurix: Membership on an entity's Board of Directors or advisory committees; Dragonfly: Membership on an entity's Board of Directors or advisory committees; Apexigen: Membership on an entity's Board of Directors or advisory committees; Viracta: Membership on an entity's Board of Directors or advisory committees; Spotlight: Membership on an entity's Board of Directors or advisory committees; Immunocore: Membership on an entity's Board of Directors or advisory committees; Walking Fish: Membership on an entity's Board of Directors or advisory committees; Kira: Membership on an entity's Board of Directors or advisory committees; Abintus Bio: Membership on an entity's Board of Directors or advisory committees; Khloris: Membership on an entity's Board of Directors or advisory committees; Virsti: Membership on an entity's Board of Directors or advisory committees; BiolineRx: Membership on an entity's Board of Directors or advisory committees; BeiGene: Membership on an entity's Board of Directors or advisory committees; Quadriga: Membership on an entity's Board of Directors or advisory committees.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal