Abstract
Background: Emicizumab is indicated for prophylaxis in persons with hemophilia A (PwHA) of all ages, with/without factor (F)VIII inhibitors (exact label may vary by country). For older persons receiving emicizumab, however, their care may be complicated by comorbidities. Prevalence and early onset of cardiovascular (CV) risk factors, including hypertension, are common among PwHA (Humphries, et al. Adv Med Sci 2018; Sood, et al. Blood Adv 2018). Human immunodeficiency virus (HIV), which is likely to be more common in older PwHA, is associated with increased risk of heart disease (Shah, et al. Circulation 2018), and co-infection of HIV with hepatitis C virus (HCV) increases the risk of end-stage liver disease and worsens HCV progression (Papadopoulos, et al. Ann Gastroenterol 2018). Data regarding emicizumab in older PwHA with comorbidities (CV risk factors or concomitant HIV and/or HCV infection) are limited. This post hoc analysis of PwHA in four Phase III studies evaluated efficacy and safety of emicizumab in PwHA aged ≥50 years with CV risk factors or HIV and/or HCV infection.
Methods: The HAVEN 1 (NCT02622321), 3 (NCT02847637), 4 (NCT03020160) and STASEY (NCT03191799) studies enrolled PwHA ≥12 years. HAVEN 1 and STASEY enrolled PwHA with FVIII inhibitors, HAVEN 3 PwHA without FVIII inhibitors and HAVEN 4 PwHA with/without FVIII inhibitors. Eligible PwHA had no history or clinical signs of cirrhosis. PwHA were excluded if they had severe hepatic disease, HIV infection with a CD4 count <200 cells/μL, or concurrent disease, treatment or abnormality that could impact safe participation (as deemed by the investigator). For this analysis, CV risk factors included past medical history of CV disease or current evidence of: hypertension, diabetes, hyperlipidemia, or obesity (body mass index ≥30 kg/m 2). HCV infection was defined as prior/current infection. Bleeds were defined per the HAVEN 1, 3 and 4 and STASEY study protocols. An age cut-off of ≥50 years was selected for this analysis. Due to overlapping subgroups, p-values were not calculable.
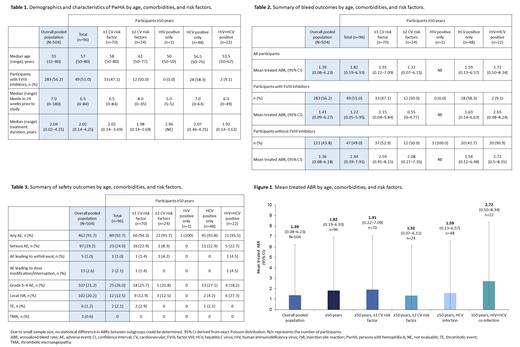
Results: At data cut-off, data were available for 504 PwHA; 96 (19.0%) were ≥50 years and eligible for this analysis. Of these, 17 were from HAVEN 1, 33 from HAVEN 3, 12 from HAVEN 4 and 34 from STASEY. The median age (range) was 57 years (50-80); 22 PwHA were ≥65 years. Median emicizumab treatment duration (range) was 2.02 years (0.14-4.25). Seventy participants (72.9%) had ≥1 CV risk factor and 24 (25.0%) had ≥2 CV risk factors. One participant (1.0%) had HIV infection, 48 (50.0%) had HCV infection, and 22 (22.9%) had HCV+HIV co-infection (Table 1).
The mean treated annualized bleeding rate (ABR) for the overall population (N=504) was 1.39 (95% confidence interval [CI]: 0.08-6.23); for eligible participants aged ≥50 years (n=96), it was 1.82 (95% CI: 0.19-6.93), and was consistent in participants with CV risk factors and HIV or HCV infection (Table 2; Figure 1). Participants aged ≥50 years with HIV+HCV co-infection (n=22) had a higher treated ABR of 2.72 (95% CI: 0.5-8.34), which included one participant with an ABR of 21 and three others with ABRs ≥5. Adverse events (AEs) and serious AEs occurred in 462 (91.7%) and 97 (19.2%) participants in the overall population and 89 (92.7%) and 23 (24.0%) of participants ≥50 years, respectively. Grade 3-4 AEs and injection site reaction rates were similar among participants in the overall population and participants ≥50 years (Table 3). Thrombotic events (TEs) and thrombotic microangiopathies occurred in six (1.2%) and three (0.6%) participants in the overall population, respectively. Of these, one TE each occurred in two participants ≥50 years (2.1%); both had ≥1 CV risk factor and one also had HCV infection.
Conclusions: ABRs for PwHA ≥50 years with CV risk factors, or with HIV or HCV infection, receiving emicizumab prophylaxis were similar to the overall study populations. Higher ABRs were observed for PwHA with HIV+HCV co-infection; however, these may have been skewed by a minority of PwHA with unexpectedly high ABRs. Furthermore, given the small sample size and wide confidence intervals, it is difficult to draw conclusions. HIV and HCV infections have been associated with increased bleeding risk in PwHA (Chen, et al. Value Health 2015), which may have had a cumulative effect in this subpopulation. Safety outcomes were similar to the overall study populations; emicizumab prophylaxis was well tolerated in PwHA ≥50 years with comorbidities.
Jiménez-Yuste: NovoNordisk: Consultancy, Honoraria, Research Funding; Grifols: Consultancy, Honoraria, Research Funding; Octapharma: Consultancy, Honoraria, Research Funding; BioMarin: Consultancy; Sanofi: Consultancy, Honoraria, Research Funding; Sobi: Consultancy, Honoraria, Research Funding; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Research Funding; Bayer: Consultancy, Honoraria, Research Funding; CSL Behring: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria, Research Funding. Oldenburg: Grifols: Consultancy, Honoraria, Speakers Bureau; Freeline: Consultancy, Honoraria, Speakers Bureau; CSL-Behring: Consultancy, Honoraria, Research Funding, Speakers Bureau; Chugai: Consultancy, Honoraria, Research Funding, Speakers Bureau; Biotest: Consultancy, Honoraria, Research Funding, Speakers Bureau; Biomarin: Consultancy, Honoraria, Speakers Bureau; Biogen Idec: Consultancy, Honoraria, Speakers Bureau; Novo Nordisk: Consultancy, Honoraria, Research Funding, Speakers Bureau; Bayer: Consultancy, Honoraria, Research Funding, Speakers Bureau; University Clinic Bonn AöR: Current Employment; Octapharma: Consultancy, Honoraria, Research Funding, Speakers Bureau; Pfizer: Consultancy, Honoraria, Research Funding, Speakers Bureau; Shire (a Takeda company): Consultancy, Research Funding, Speakers Bureau; Roche: Consultancy, Speakers Bureau; Sobi: Consultancy, Speakers Bureau. Tzeng: Genentech, A Member of the Roche Group: Current Employment; Genentech/Roche: Current equity holder in publicly-traded company, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company, Divested equity in a private or publicly-traded company in the past 24 months. Trzaskoma: Genentech, Inc.: Current Employment, Current equity holder in publicly-traded company, Current holder of stock options in a privately-held company. Sanabria: F. Hoffmann-La Roche Ltd: Current Employment, Current holder of individual stocks in a privately-held company. Mahlangu: Spark: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novo Nordisk: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Biomarin: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Baxalta: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; CSL Behring: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Catalyst Biosciences: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Univeristy of the Witwatersrand: Current Employment; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Novartis: Research Funding; Pfizer: Research Funding; Unique: Research Funding; Sanofi: Research Funding, Speakers Bureau; Takeda: Speakers Bureau; WFH: Speakers Bureau; ISTH: Speakers Bureau; Springer: Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal