Abstract
Introduction:
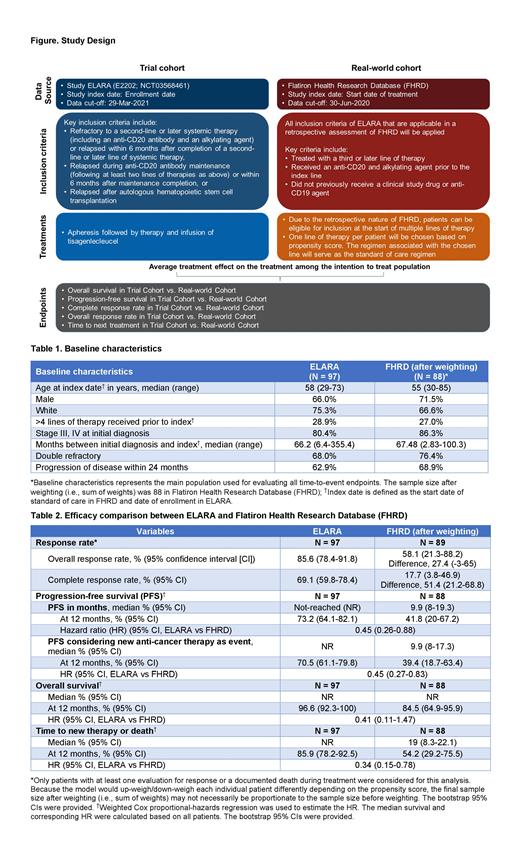
In the single-arm phase 2 ELARA trial (NCT03568461), tisagenlecleucel demonstrated efficacy and favorable safety profile in patients with relapsed/refractory (r/r) follicular lymphoma (FL) after ≥ 2 lines of prior therapy, including in high-risk sub-populations (Schuster, et al. ASCO 2021). To contextualize these results, we performed a retrospective non-interventional study to compare the efficacy of tisagenlecleucel seen in the single-arm ELARA trial with the standard-of-care (SoC) using individual patient-level data from the US Flatiron Health Research Database (FHRD). FHRD is a database derived from electronic health records from over 280 cancer clinics. The objective was to assess the effect of prescribing tisagenlecleucel vs SoC in patients who participated in ELARA.
Methods:
Individual patient-level data from FHRD were used to create an external control arm to carry out an indirect comparison with the ELARA trial. Eligible inclusion and exclusion criteria from ELARA were applied to the external control arm. A single eligible line was selected using propensity scores when patients were qualified at multiple lines.
Key prognostic factors including age, race, gender, number of prior treatment lines, group stage at initial FL diagnosis, number of months between initial FL diagnosis and indication of index treatment, double refractoriness, and disease progression within 24 months were included in a propensity score model to reduce confounding due to systematic differences in ELARA patients from FHRD patients at baseline for the selected line. Weighting by odds of receiving tisagenlecleucel was used to estimate the average treatment effect on progression-free survival (PFS), overall survival (OS), time to next treatment (TTNT), overall response rate (ORR), and complete response rate (CRR).
The rates and difference in rates were calculated for CRR and ORR. Kaplan-Meier (KM) analysis and Cox proportional hazards model were used to analyze all time-to-event endpoints. 95% confidence interval (CI) was calculated using bootstrapping. Data from the first 24 months after enrollment in ELARA or after treatment in FHRD were used for the follow-up period in the time to event endpoints, as few patients in ELARA trial had > 24 month data (Figure). Results were reported based on the post-weighting sample by incorporating a weight factor in the above analyses. A series of sensitivity analyses were conducted to assess the robustness of the primary analysis.
Results:
As of Mar 29, 2021, 98 patients were enrolled in the ELARA trial, of which 97 were included in this indirect comparison (median follow-up, 15 months). In the FHRD cohort (data cut-off, Jun 30, 2020), 98 patients with ≥ 3 treatment lines who met the ELARA eligibility were included (median follow-up, 14 months in the post-weighted sample) (Table 1).
In the ELARA vs FHRD cohorts, after applying weighting by odds, the ORR was 85.6% vs 58.1%, and the CRR was 69.1% vs 17.7%. The difference in CRR (51.4%; 95% CI: 21.2, 68.8) was clinically meaningful (Table 2).
The median TTNT or death was not reached in the ELARA cohort and was 19.0 months in the FHRD cohort after weighting (HR = 0.34 [95% CI: 0.15, 0.78]) (Table 2).
The median OS was not reached for both ELARA and FHRD cohorts in the first 24-month period. KM estimate of OS at 12 months was 96.6% in the ELARA cohort and 84.5% in the FHRD cohort, post weighting. The estimated 59% risk reduction was in favor of tisagenlecleucel over SoC (hazard ratio [HR] = 0.41 [95% CI: 0.11, 1.47]) (Table 2).
The median PFS was not reached in the ELARA cohort and was 9.9 months in the FHRD cohort, after weighting. In the ELARA vs FHRD cohorts, the 12-month PFS was 73.2% vs 41.8%, with a HR of 0.45 indicating a 55% reduction in the risk of progression and death with tisagenlecleucel vs SoC. The median PFS considering new anti-cancer therapy as an event was not reached for ELARA and was 9.9 months for FHRD; the estimated probability of being progression-free at 12 months was 70.5% in the ELARA cohort and 39.4% in the FHRD cohort (Table 2). Sensitivity analyses results were consistent with that of primary analysis.
Conclusion:
In the weighted analyses with adjustment for baseline prognostic factors, there was a consistent trend towards greater CRR, TTNT, OS and PFS in favor of tisagenlecleucel vs SoC in patients with r/r FL. These results support the clinically meaningful treatment benefit of tisagenlecleucel observed in the ELARA trial.
Hao: Novartis: Current Employment. Hsu: Novartis: Consultancy. Parzynski: Novartis: Consultancy. Lobetti Bodoni: Spouse: Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Spouse: Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Spouse: Celgene: Honoraria; Spouse: Harlcok Healthcare: Current holder of individual stocks in a privately-held company; Spouse: Takeda: Consultancy, Honoraria, Speakers Bureau; Spouse: NHS: Ended employment in the past 24 months; Spouse: Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees; Spouse: F. Hoffmann-La Roche: Current Employment, Current equity holder in publicly-traded company; Gilead: Other: Travel sponsorship in June 2019; Novartis: Current Employment, Current equity holder in publicly-traded company. Degtyarev: Novartis: Current Employment, Current equity holder in publicly-traded company. Hampson: Novartis: Current Employment. Masood: Novartis: Current Employment, Current holder of stock options in a privately-held company. Wu: Novartis: Consultancy.
Tisagenlecleucel (Kymriah) an autologous CD19-directed CAR-T-cell therapy, has been approved for children and young adults with relapsed/refractory (r/r) acute lymphoblastic leukemia and, adults with r/r diffuse large B-cell lymphoma.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal