Abstract
Introduction: Treatment Free Remission (TFR) is the ultimate goal of therapy for most CML patients. Despite adopting consensus eligibility criteria of a sustained deep molecular response and more than 4 years of TKI therapy, the relapse rate after TKI cessation is still around 50%. More sensitive detection of residual leukaemia has the potential to improve our capacity to predict TFR outcomes for individual patients.
Aim: To correlate droplet digital PCR (ddPCR) assay results with TFR outcome, especially in the setting of undetectable levels measured by qRT-PCR.
Method: ddPCR was performed on blood samples from 51 TFR-eligible CML patients at the time of TKI cessation. 5 µg RNA per sample was used in 8 wells/sample using the BioRad QXDx BCR-ABL %IS kit on QX200 ddPCR system which yielded BCR-ABL1% (IS) directly. All these patients achieved MR4.5 that was sustained for ≥ 2 years.
Results: 100% of patient were in MR4.5 via qRT-PCR at the time of stopping. 61% of the 51 patients evaluated relapsed within 12 months. Median duration of TKI therapy for the whole group was 5.8 years (range 2.2- 14 years). 20 patients achieved TFR success with a median follow up of 24 months (TFR group; sustained BCR-ABL1 <0.1% (IS) after TKI discontinuation for ≥12 months), while 31 patients relapsed (Relapse group; BCR-ABL1 >0.1% (IS) after stopping; median time of relapse 3 months, range 1-10 months).
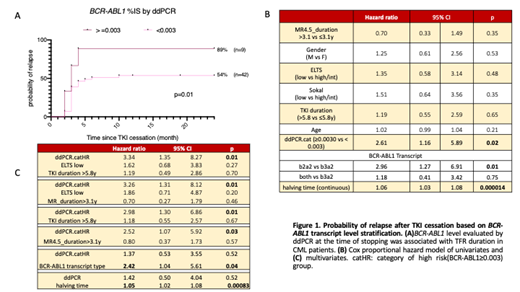
A ROC curve analysis correlated TFR outcome with ddPCR results, with BCR-ABL1 level ≥0.003% via ddPCR at the time of stopping identified as an optimal cut-off. Kaplan-Meier analysis showed that 89% of the patients with ddPCR ≥0.003 relapsed after TKI cessation, whereas the ddPCR <0.003 demonstrated a significantly reduced relapse rate to 54% (p=0.01, Figure 1A). In addition, the TFR group (median BCR-ABL1 0.00065%) demonstrated approximately two-fold lower levels of BCR-ABL1transcript level compared to the relapse group (median 0.0012%). Interestingly, 7/31 (23%) of the relapsed group had undetectable BCR-ABL1 transcript even with the current highly sensitive method, while this undetectable level was only observed in 35% of the TFR group.
We next assessed other known predictors of TFR success relative to ddPCR results in a Cox proportional hazard model. We have previously demonstrated that the BCR-ABL1 halving time after commencing therapy is highly predictive of TFR. At a univariate level, transcript type (e13a2 versus e14a2, p=0.01), BCR-ABL1 halving time (p>0.0001), and mRNA quantitation by ddPCR ≥ 0.003% (p=0.02) were all significantly associated with clinical outcome. Other variables including gender, age, ELTS score, Sokal score, MR4.5 duration and TKI duration were not associated with clinical outcome in this cohort (Figure 1B). In the multivariate analyses (Figure 1C), ddPCR remained an independent predictor after adjusting for ELTS, TKI duration and MR4.5 duration. Interestingly, ddPCR was not an independent predictor after adjusting for BCR-ABL1 transcript type or halving time.
Conclusion: QXDx ddPCR assay is a promising tool for molecular residual disease monitoring in CML, especially when the BCR-ABL1 is undetectable by conventional method. The CML patients with levels of detectable BCR-ABL1 ≥0.003% measured by ddPCR have a significantly higher probability of relapse compared to patients with lower levels of the transcript. The ≥0.003% BCR-ABL1 level cut-off value could be a potential tool to aid decision-making when attempting TKI discontinuation in CML. However, even though a measurable level of BCR-ABL1 above 0.003% via ddPCR identified patients at high risk of relapse after a TFR attempt, it does not rule out the possibility of TFR; and a negative ddPCR result does not exclude the risk of molecular relapse. ddPCR may be most useful where other TFR predictive factors including BCR-ABL1 transcript type and halving time are not available.
In-kind support was received from Bio-Rad for this study.
Branford: Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Cepheid: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Qiagen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Hughes: Novartis: Honoraria, Research Funding; Incyte: Honoraria; BMS: Honoraria, Research Funding. Yeung: BMS: Honoraria, Research Funding; Amgen: Honoraria; Novartis: Honoraria, Research Funding; Pfizer: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal