Abstract
Background: Germline predisposition is increasingly being recognised in myeloid neoplasms (MN) including primary myelodysplastic syndrome. An unequivocal diagnosis of germline predisposition carries actionable considerations for patient management including donor stem cell source for allogeneic transplantation, dose-reduction of conditioning regimes and screening for extra-hematological disease (such as pulmonary abnormalities in patients with telomere biology disorders). In addition, the identification of MDS predisposition syndromes can avoid misdiagnosis (for example, distinguishing idiopathic thrombocytopenic purpura from thrombocytopenia due to RUNX1 germline variant). However, the prevalence of pathogenic germline variants (PGVs) in unselected pMDS patients presenting at older age remains unknown.
Aim: This study assesses frequency and type of pathogenic germline variants in MDS patients and compares with age matched healthy controls and patients with other cancers.
Method: We analysed 68 known cancer predisposition genes in germline samples of 146 samples from myeloid neoplasms. Study included primary MDS (n=51) and MDS diagnosed in cancer survivors with (n=77) or without prior exposure to cytotoxic therapy (n=18). Using uniform American College of Medical Genetics and Genomics (ACMG) guidelines for annotating germline mutation, we also compared the frequency of pathogenic germline variants in the same genes with patients with single cancer and age-match healthy controls (>70 years).
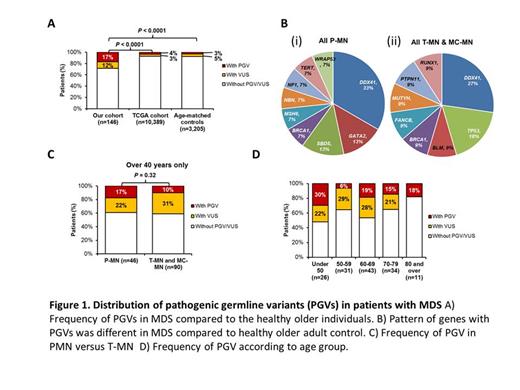
Results: Pathogenic germline variants (PGVs) were identified in 19% (28/146) patients compared to 4% and 3% patients with single cancer and age-matched controls respectively (P<0.0001) (Fig. 1A). Median age at diagnosis was similar between MN patients with or without PGVs [66 years (19-81) vs. 70 years (33-87); P=0.06]. PGVs were most frequent in DDX41 (n=7, 33%) followed by BRCA1 (n=2, 10%), GATA2 (n=2; 10%) and TP53 (n=2; 10%) (Fig.1B). We also identified pathogenic copy number variations (CNV) in 4 patients. The distribution of PGVs was also different, with DDX41 PGVs absent in single cancers and more prevalent in MN than age-matched controls (35% vs. 4%, P<0.001). The frequency of PGV was not significantly different between P-MN and T-MN/ MC-MN (17% versus 10%, P = 0.32 (Fig.1C). The frequency of PGV was 30%, 6%, 19%, 15% and 18% in patients ≤50, 51-59, 60-69, 70-79 and >80 years of age (Fig. 1D).
Phenotypic features such as monocytopenia and mycobacterium infections (MonoMAC; SA460) and personal and family history of pulmonary fibrosis (SA918) were present in only two cases with PGVs. Family history of MDS/AML was present in only in four cases with PGVs, in which PGVs were found in typical myeloid malignancy genes (DDX41, GATA2). Importantly, some patients with family history of solid cancers carried PGVs in genes traditionally associated with solid cancers (e.g. MSH6, NF1, TP53 and BRCA1). SA927 had a PGV in MSH6 and multiple first-degree relatives with solid cancers including colon, renal and brain cancers. Moreover, 41% of adults with hematological disorders and a personal and/or family history of interstitial lung disease had PGVs in telomere biology disorder genes. Hence, family history should not be restricted to hematological disorders, but also solid cancers and non-malignant phenotypes (e.g. hepatic and pulmonary fibrosis). The frequency of PGVs was not different in patients with and without family history of cancer (23% vs. 13%, P=0.32).
Conclusion: The frequency of PGVs is significantly high in MN compared to age matched healthy control and other cancer patients. Our observation of a high frequency of PGVs in the older MDS population warrants standardization of germline testing at diagnosis to guide optimal management of patients and their families.
Hiwase: Novartis: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal