Abstract
Background: The incidence of B-cell ALL follows a bimodal distribution, with the first peak occurring in childhood and a second peak occurring around the age of 50. While the prognosis for pediatric patientshas improved over the past decades, prognosis for the elderly remains very poor. Despite a high rate of response to induction chemotherapy, only 30-40% of adult patients with ALL will achieve long-term remission. Patients with primary refractory and chemo-refractory B-cell ALL have an extremely poor prognosis.
A chimeric antigen receptor (CAR) specific for the CD19 B lymphocyte molecule has provide an option of treating B-cell malignancies. Although these CAR-modified T cell products target the same antigen, the designs of CD19-targeted CAR T-cells vary significantly. CNCT19 is autologous CD19-directed genetically modified T cell immunotherapy. The structure of chimeric antigen receptor (CAR) includes CD19scFv-hCD8α hinge-CD8α TM-4-1BB-CD3ζ in tandem. The patent protected CD19scFv sequence is derived from CD19 monoclonal antibody HI19a featured with high specificity and affinity to CD19. This strong binding capability to CD19 leads to killing of the CD19 positive malignant B-cells. Use of 4-1BB co-stimulatory domain is expected to reduce the severity of treatment-associated cytokine release syndrome (CRS) and neurologic toxicities (NT) while maintaining a stronger and longer-term anti-tumor effect.
Methods: CNCT19 has been investigated in an open-label, phase 1/2 clinical trial for adult and pediatric patients with r/r ALL who have failed ≥ 2 lines prior therapies. Leukapheresis to obtain T cells was performed following the SOP of the local hospitals. CNCT19 was infused 2 to 14 days after lymphodepletion. All the patients were hospitalized up to 2 weeks. All the PIs have been trained for the management of CNCT19-treatment related CRS and neurotoxicities. A PI committee was involved in the management of CRS and neurologic toxicity. The primary objectives of the study were to determine the safety and the clinical outcomes, including ORR within 3 months, PFS, and overall survival (OS).
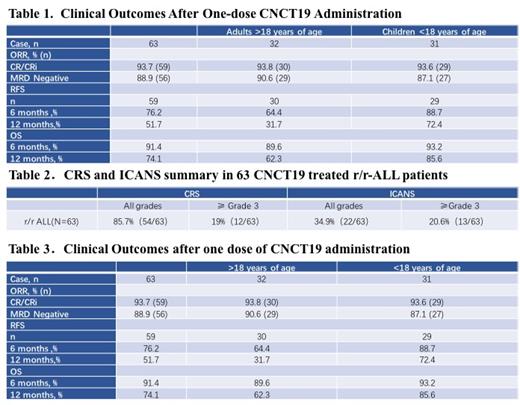
Results: As of October 2020, 63 patients (adults: n=32 >18 years, children: n=31 < 18 years) have received CNCD19 treatment and all subjects have been followed at least for one year.
All grade adverse events occurred in 54 patients (54/63 = 85.7%). Grade ≥3 CRS and NEs occurred in 12 (12/63 = 19%) and 13 (13/63 = 20%), respectively. Out of 63 patients, 59 (59/63= 93.6%) have had overall responder rate (ORR) defined as complete remission (CR) and complete remission with incomplete hematologic recovery (CRi). Among the 59 responded patients, the minimal residual disease (MRD) was negative in 56 patients (56/69 = 94.9%) and 23 patients (38.9%) received allogeneic-HSCT treatment. The relapse-free survival (RFS) and overall survival (OS) were summarized in adult and pediatric groups separately in the following table.
Conclusions:
CNCT19 treatment of patients with r/r ALL demonstrates improved ORR, PFS, and OS both in children and adults. A pivotal clinical trial is going on in China to evaluate the ORR, PFS, and OS with a clinical plan to decrease the severity of treatment-related adverse events (CRS/NE).
Wang: AbbVie: Consultancy; Astellas Pharma, Inc.: Research Funding.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal