Abstract
Background: Patients with low-risk myelodysplastic syndrome (MDS) and aplastic anemia (AA) often need transfusions, which may accelerate the iron overload. The aim of this study was to evaluate the efficacy, safety and dose-effect relation of deferasirox (DFX) for patients with low-risk MDS and AA who were refractory to regular treatment in the real-world setting.
Methods: This was a retrospective study. Patients with low-risk MDS or AA who failed to standard treatments and were transfusion-dependent were enrolled. DFX was given as the only treatment apart from transfusion. Patients were recorded for their medical history, laboratory tests, nuclear magnetic resonance (MRI), echocardiography and calculated for their overall survival (OS). Dose-effect relations of DFX were evaluated after the first 6 months. Total annual exposure of DFX was calculated after 12 months, expressed as accumulated exposure time at dose of 20mg/kg/d.
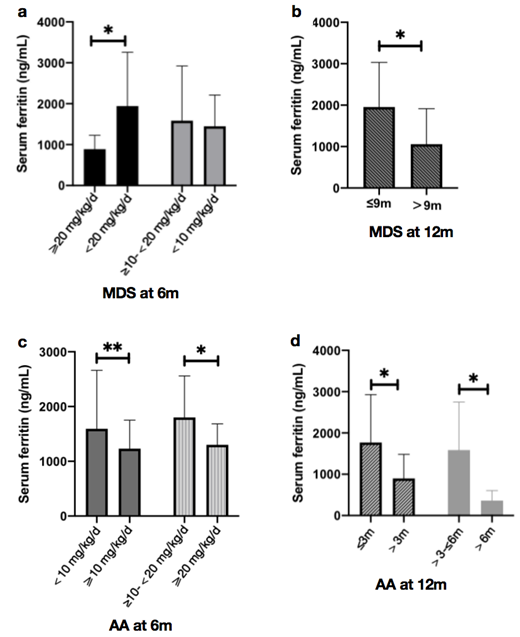
Results: Of the 112 patients finally enrolled, 61 (54.5%) were low-risk MDS and 51 (45.5%) were AA. The median age was 56 (10, 89) years and 52.7% patients were males. The minimum dose of DFX for significant SF decrease was 20 mg/kg/d at 6 months; and the minimum accumulation of DFX had to reach 9 months at 20 mg/kg/d to maintain the efficacy at the time of 12 months for patients with low-risk MDS (p<0.050). Different from MDS, the minimum dose for significant SF decrease was 10 mg/kg/d at 6 months; and the minimum accumulation had to reach 3 months at 20 mg/kg/d to maintain the efficacy at the time of 12 months for patients with AA (p<0.050). Meanwhile, with same dose of exposure, significant improvements in hematological parameters were also observed in AA, but no dose-effect relations were found in MDS. 62.3% MDS and 51.0% AA patients stopped transfusion in the next 6 months. Erythroid responders had lower SF than non-responders after 12 months of DFX, both for MDS and AA (p<0.05). Lower alanine aminotransferase (ALT) and aspartate aminotransferase (AST) compared with baseline after 12 months of DFX were observed and longer exposure time correlated with lower ALT (p<0.050). No significant changes in cardiac function, however. Similar side effects were found in MDS and AA, with gastrointestinal disorders and elevated serum creatinine the most common. Higher dose and longer exposure time of DFX correlated with longer overall survival, both for patients with MDS and AA (p<0.050).
Conclusion: Although dose of DFX varies greatly in different individuals, a significant decrease in SF and an improvement of hematologic parameters, organ functions, or even overall survival can be achieved if the accumulate dose reaches a certain level. In that case, patients with low-risk MDS need higher dose than those with AA.
No relevant conflicts of interest to declare.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal