Abstract
Introduction
Sickle cell disease (SCD) affects 1 in 365 African Americans and approximately 25 million people world-wide. A common skeletal system complication is avascular necrosis (AVN), which can cause substantial pain and a reduced quality of life. While early management of AVN is focused on increasing range of motion with physical therapy and pain relief, there are no clear predictors for who is more likely to develop AVN and earlier institution of these preventive measure could help decrease disease progression. Vascular endothelial growth factor (VEGF) is a biomarker of endothelial injury and may indicate reduced vascular supply to the femoral or humeral head. Here we describe potential risk factors and biologic pathways for AVN in SCD, as understanding these may lead to improvements in future monitoring, early detection, and early intervention practices.
Methods
We investigated clinical and laboratory risk factors associated with AVN in a cohort of 435 SCD patients from our center. Blood samples, clinical, and laboratory data were collected at the time of enrollment during a clinic visit. Genotyping for alpha thalassemia was performed by PCR and the serum concentration of VEGF was measured by ELISA. AVN status was confirmed by review of the medical record and available imaging. We conducted a cross-sectional analysis comparing categorical and linear variables by AVN status using the chi-square and Kruskal-Wallis test, respectively. The independent association of the clinical and laboratory variables with AVN status was determined by logistic regression analysis. The initial model included variables with a P-value < 0.1 on univariate analysis and the final model was ascertained by stepwise forward and backward selection. Median values and interquartile range (IQR) are provided.
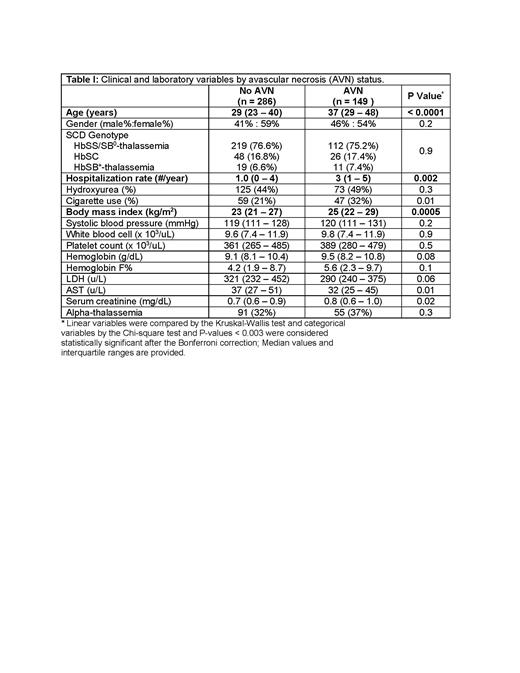
Results
The median age of the cohort was 32 (IQR, 24 - 43) years, 57% (250/435) were female, and 46% (198/435) were on hydroxyurea. AVN was observed in 34% (149/435) of SCD patients. SCD patients with AVN were older, had more frequent vaso-occlusive crises requiring medical attention, and had a higher body mass index (Table I) (P ≤ 0.002). We measured VEGF in 241 of the SCD patients with serum samples available at the time of enrolment. Serum VEGF concentrations trended higher in SCD patients with versus without AVN (420 vs. 359 pg/mL, respectively; P = 0.078). In the multivariate analysis model, AVN was independently associated with increased number of vaso-occlusive crises (OR 1.1, 95% CI: 1.0 - 1.14; P = 0.02), AST concentration (natural log OR 0.5, 95% CI: 0.2 - 0.9; P = 0.03), VEGF concentration (natural log OR 1.4, 95% CI: 1.0 - 1.9; P = 0.047), and tobacco use (OR 1.9, 95% CI: 0.9 - 3.7; P = 0.078).
Discussion
In conclusion, we demonstrate a high prevalence of AVN in an adult cohort of SCD patients. The presence of AVN was independently associated with a greater frequency of vaso-occlusive pain episodes, which may demonstrate a shared pathophysiology between AVN and vaso-occlusion that merits further investigation. We demonstrate that serum VEGF concentrations are higher in SCD patients with AVN and may be a clinical tool to identify those at high-risk and for earlier intervention for this complication.
Gordeuk: Modus Therapeutics: Consultancy; Novartis: Research Funding; Incyte: Research Funding; Emmaus: Consultancy, Research Funding; Global Blood Therapeutics: Consultancy, Research Funding; CSL Behring: Consultancy. Saraf: Pfizer: Research Funding; Global Blood Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal