Abstract
Background: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) induced coronavirus disease-2019 (COVID-19) has presented humanity with unprecedented challenges. Severe disease is associated with acute respiratory distress syndrome (ARDS), use of mechanical ventilation, ICU stay and prolonged hospitalization, The role of the immune system in the pathogenesis of COVID-19 disease is still unclear, which imposes limitations on identifying potential immunotherapy that can improve care for acute and chronic phases of COVID-19 in conjunction with current therapies. Research efforts are ongoing for more than 1 year to identify key immunological mechanisms involved in the disease process. While insightful, this knowledge is still incomplete and can be complemented with the assessment of immune response kinetics. Such assessment will help with the identification of early interventional modalities of immune cell regulation. With these considerations in mind, we aimed to assess several parameters of immune system regulation during the current medical care of patients with COVID-19.
Methods: This is a pre-clinical prospective cohort study which involved laboratory-based assessments of blood samples obtained from COVID-19 patients and healthy volunteers. The study population was divided into three cohorts. Our first cohort included 18 years and older COVID-19 patients with respiratory complaints, oxygen (O2) saturations of less than or equal to 92 and pulmonary infiltrates on an imaging study or who were critically ill and required ventilatory support. Second cohort included 18 years and older COVID-19 patients who were hospitalized and did not require ventilatory support. Third cohort included participants with no prior diagnosis of COVID-19, or any recent viral respiratory symptoms including fever, cough or shortness of breath for the last 2 weeks. Patients with an established diagnosis of cancer or immunologic disorders were excluded. Blood specimens were collected over the period of hospitalization: specimen number 1 on day 1-3 of hospitalization, specimen number 2 on days 3-4 of hospitalization, specimen number 3 on days 5-7 of hospitalization, and specimen number 4 on 7-30 days after discharge. We performed capillary electrophoresis for serology and automated ELISA for cytokine measurement. We collected clinical data on patient demographic, clinical characteristics such as presence of any acute and chronic comorbidities and serum inflammatory markers C-Reactive Protein, D-Dimer and Ferritin.
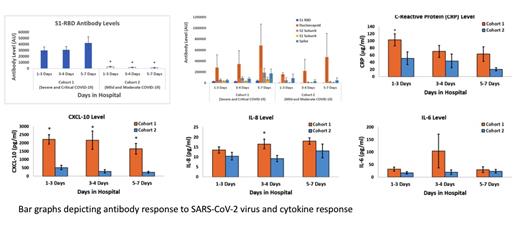
Results: We had 15 patients in cohort 1, 10 in cohort 2 and 15 in cohort 3. Patients in cohort 1 were older and had higher comorbidities. Males constituted a substantially high percentage of patients in cohort 1 and 2 (60% and 70% respectively). Patients had similar BMI in cohort 1 and 2. Total antibody levels were highest in cohort 1 but an upward trend over the course of hospitalization was noted in all cohorts. Most interesting pattern was noted in the context of antibodies against spike protein S1 receptor-binding domain (S1RBD) where patients in cohort 2 developed minimal S1RBD antibodies. Cohort 1 on average had higher levels of Interleukin 6(IL-6), Interleukin 8(IL-8), C-X-C motif chemokine ligand 10 (CXCL10) and other inflammatory cytokines except Interferon gamma (IFN-gamma) compared to Cohort 2. Remarkable difference in CXCL-10 levels was noted between the groups and healthy volunteers had the lowest levels. No significant difference in IFN-gamma was noted between cohorts and the levels quickly depleted over the course of the infection.
Conclusion: Our analysis confirms that neutralizing antibodies do not correlate with lessened COVID-19 disease severity. Severe COVID-19 infection is secondary to ineffective innate immunity associated with immune overshoot. CXCL10 serves as a major component in triggering the cytokine storm that is a hallmark of SARS-CoV-2 infections. Our findings show an association between high levels of CXCL10 and more severe COVID-19 infection. There does not seem to be any significant correlation with disease severity and IFN-gamma levels.
No relevant conflicts of interest to declare.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal