Abstract
Background: Apixaban is a treatment option for venous thromboembolism (VTE) in cancer patients. There are no data on the effect of low dose apixaban after 6 months treatment. We wanted to assess the efficacy and safety of apixaban 2.5 mg twice daily as prophylaxis for recurrent VTE after 6 months initial treatment with full-dose apixaban.
Patients and methods: We included 298 patients with cancer and any type of VTE. All patients were treated with full dose apixaban for the first 6 months. After 6 months, all patients with active cancer continued with apixaban 2.5 mg twice daily and were followed for the next 30 months. The primary endpoint of efficacy was recurrent VTE, the primary safety endpoint was major bleedings. Clinically relevant non-major bleedings was a secondary endpoint. The endpoints are reported as incidence rates or fractions with 95% confidence intervals, and as Kaplan-Meier plots.
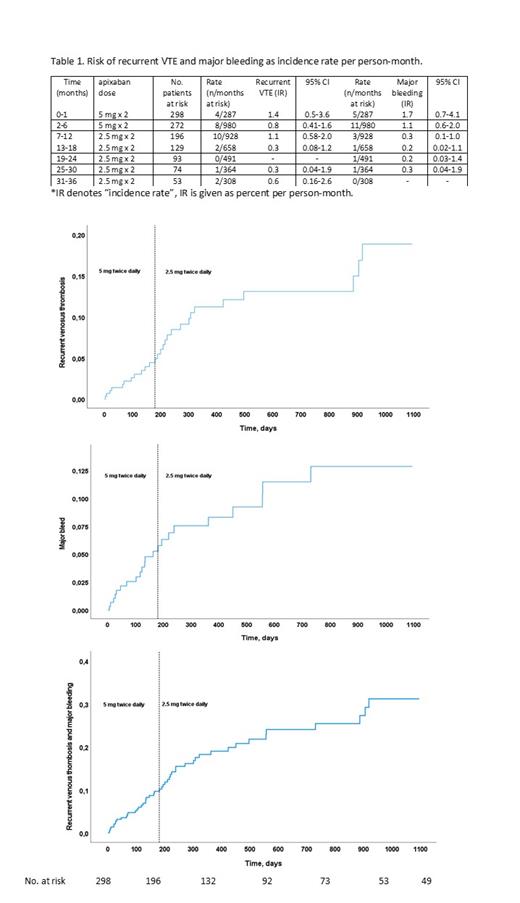
Results: During the first 6 months of full-dose anticoagulation 12 of 298 patients had recurrent VTE (4.0%, 95% confidence interval 2.1-6.9), 16 experienced major bleeding (5.4%, 95% CI 2.8-7.9%), and 26 patients experienced one or more episodes of CRNMB (8.9%, 95% CI 5.5-12) as previously reported. 1 Of the 298 patients included, 196 continued with apixaban 2.5 mg twice daily after 6 months. During treatment from 6 to 36 months with low-dose apixaban 15 of 196 (7.6%, 95% CI: 4.4-12) patients had recurrent VTE, 7 (3.6%, CI: 1.5-7.2) patients experienced major bleeding and 16 (8.2%, 95% CI: 4.7-13) patients experienced CRNMB. The highest incidence rate of both recurrent VTE and major bleedings were seen during the first month of full-dose apixaban (Table 1). After the dose reduction of apixaban, the incidence rate of recurrent VTE increased slightly during 6 to 12 months while the incidence rate of major bleeding decreased during the same time-period. After 12 months the incidence rate of both recurrent VTE and major bleeding was low and remained low during the entire 30 months follow-up (Table 1 and Figure 1). The Kaplan-Meier plot of the composite endpoint of recurrent VTE or major bleeding did not change after dose-reduction. After about 9 months treatment (i.e. 3 months on low dose apixaban) the Kaplan-Meier curve of the composite endpoint flattened out.
Conclusion: Dose reduction of apixaban to 2.5 mg twice daily after 6 months of full dose anticoagulation resulted in a small increase in recurrent VTE, but a marked decrease in major bleedings during the 6-12 months period. After approximately 9 months the frequency of recurrent VTE and major bleedings remained low compared with the first 6 months of full-dose treatment. Reducing the dose of apixaban to 2.5 mg twice daily after 6 months of full-dose treatment appears safe and effective.
References
1. Hannevik TL, Brekke J, Enden T, et al: Thrombosis and bleedings in a cohort of cancer patients treated with apixaban for venous thromboembolism. Thromb Res, 2020
Hannevik: Pfizer/Bristol Myers Squibb: Membership on an entity's Board of Directors or advisory committees. Garresori: Pfizer: Honoraria; Amgen: Honoraria; Bayer: Honoraria. Froen: Bristol-Myers Squibb: Honoraria; Amgen: Membership on an entity's Board of Directors or advisory committees. Porojnicu: Bristol-Myers Squibb: Honoraria. Ghanima: Bayer, BMS/Pfizer: Research Funding; Amgen, Novartis, Pfizer, Bristol Myers Squibb, SOBI, Griffols, Sanofi: Honoraria; Amgen, Novartis, Pfizer, Principia Biopharma Inc- a Sanofi Company, Sanofi, SOBI, Griffols, UCB, Argenx: Consultancy. Dahm: Pfizer: Honoraria; Novartis: Honoraria; Pfizer/Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal