Abstract
Acute myeloid leukemia (AML) is characterized by the heterogeneous clonal expansion of undifferentiated myeloid cells in the bone marrow (BM). AML cells compete with normal hematopoietic cells and rewire the BM microenvironment into niches that selectively support leukemia stem cells (LSC). The leukemic niche produces soluble factors that facilitate the retention of LSC and provide protection from cytotoxic and targeted agents. The vascular adhesion molecule, E-selectin is expressed on endothelial cells (EC) in the perivascular niche where therapy-resistant AML cells have an increased affinity to E-selectin compared to normal hematopoietic stem cells (HSC) (Winkler et al., 2020). We previously demonstrated (Chang et al., ASH 2020) that E-selectin blockade by the pharmacological antagonist, GMI-1271 (uproleselan; GlycoMimetics, Inc) sensitized therapy-resistant LSC to Bcl-2 targeted therapy. Efficacious eradication of LSC in the BM however requires blocking multiple receptors and/or associated signaling pathways. A more optimal dislodgement of LSC from the BM could be attained by combining an E-selectin antagonism with blockade of the CXCR4/SDF-1α axis. The dual antagonist of E-selectin and CXCR4, GMI-1359 (GlycoMimetics, Inc.), has been tested in a phase 1 clinical trial (NCT02931214). Previously, we showed that GMI-1359 in combination with a FLT3-ITD inhibitor, improved survival in a xenograft model of FLT3-ITD + AML (Zhang et al., 2016). Hence, we hypothesized that co-targeting E-selectin/CXCR4 more efficiently mobilizes AML cells from BM niches and synergizes with the anti-leukemia activity of venetoclax/hypomethylating agent (Ven/HMA).
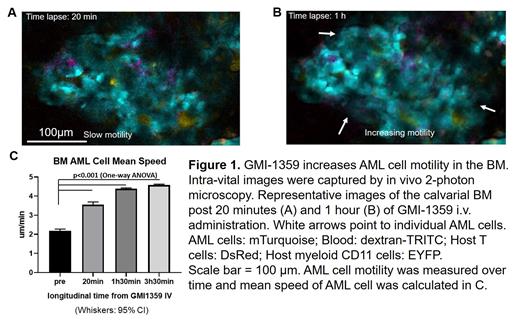
Intra-vital 2-photon imaging and tracking of individual leukemia cells in triple reporter mice (Blood: dextran-TRITC; Host T-cells: DsRed; Host myeloid CD11 cells: EYFP) injected with AML cells carrying a turquoise fluorescent protein reporter gene suggested that dual inhibition of E-selectin/CXCR4 with GMI-1359 significantly enhanced AML cell motility (Fig 1. from 2.2 um/min to 5.4 um/min, p<0.001). Individual cells were dislodged from the niche and traveled long-distance. The combined inhibition of E-selectin and CXCR4 depleted BM leukemia cells in vascular niche areas. In a patient-derived primary AML xenograft (PDX) model (harboring mutations in JAK2 and c-Kit), combinatorial treatment of GMI-1359 with Ven/HMA significantly reduced BM retention of LSC compared to control cohorts or to Ven/HMA alone (p = 0.02 and p=0.003, respectively).
In order to better understand how the augmented AML mobilization improves the efficacy of AML therapy, BM cells from PDX mice treated for 2 weeks with GMI-1271, GMI-1359, Ven/HMA, and their combinations were analyzed by single-cell proteomics (CyTOF). Blockade of E-selectin alone or dual E-selectin/CXCR4 inhibition in combination with Ven/HMA diminished levels of E-selectin ligand, mTOR, pFAK, pRb, cMyc, while increasing p21 and cleaved caspase3, which was associated with significant reduction of BM-resident LSC compared to Ven/HMA alone (CD45+34+CD38-CD123+, p= 0.03). AML blasts from the BM of the combinatorial treatment groups showed altered signaling including decreased Ki67, pRb, pNFkB, pPI3K, and E-selectin ligand, and increased levels of cleaved caspase 3. We further found that Ven/HMA significantly diminished CD31+ EC in the BM compared to control cohorts (p= 0.009). However, pharmacological antagonists of E-selectin or E-selectin/CXCR4 protected EC from Ven/HMA-induced detrimental insults through upregulation of survival signaling cascades including pAKT, pERK, pMAPK and decreased eNOS expression in EC compared to Ven/HMA treatment alone. Both EC and MSC were protected by dual inhibition of E-selectin/CXCR4 with GMI-1359. We also observed upregulated pro-survival signaling pathways such as phosphorylation of AKT-MAPK-ERK along with increased Bcl-xL, Bcl-2, and Idu expression in MSC from the GMI-1359 + Ven/HMA treated PDX mice compared to Ven/HMA single treatment cohorts.
Collectively, our results provide strong evidence that co-targeting E-selectin/CXCR4 with GMI-1359 profoundly reduces BM retention of LSC as well as protects BM niche component cells from apoptosis induced by targeted therapy, resulting in improving the anti-leukemia activity of Ven/HMA therapy in AML.
Fogler: GlycoMimetics Inc.: Current Employment, Current equity holder in publicly-traded company, Patents & Royalties. Magnani: GlycoMimetics Inc.: Current Employment, Current equity holder in publicly-traded company, Patents & Royalties. Carter: Ascentage: Research Funding; Syndax: Research Funding. Andreeff: Oxford Biomedica UK: Research Funding; ONO Pharmaceuticals: Research Funding; AstraZeneca: Research Funding; Reata, Aptose, Eutropics, SentiBio; Chimerix, Oncolyze: Current holder of individual stocks in a privately-held company; Karyopharm: Research Funding; Breast Cancer Research Foundation: Research Funding; Syndax: Consultancy; Daiichi-Sankyo: Consultancy, Research Funding; Novartis, Cancer UK; Leukemia & Lymphoma Society (LLS), German Research Council; NCI-RDCRN (Rare Disease Clin Network), CLL Foundation; Novartis: Membership on an entity's Board of Directors or advisory committees; Amgen: Research Funding; Aptose: Consultancy; Glycomimetics: Consultancy; Medicxi: Consultancy; Senti-Bio: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal