Abstract
Background: Differences in outcomes between clinical trial and real-world populations in follicular lymphoma (FL) may be partially explained by differences in characteristics between clinical trial and real-world patients. We hypothesized that certain eligibility criteria may be responsible for populations with distinct demographic/clinical characteristics. In this study, we compiled a list of eligibility criteria from front-line FL trials and evaluated their impact on the potentially eligible FL patients derived from two national cohort studies. We also evaluated whether criteria were duplicative and whether liberalizing criteria might enlarge the pool of eligible patients.
Methods: Eligibility criteria were abstracted from 21 first-line clinical trials in FL in the FLASH database. We identified FL patients in two prospective cohort studies: Molecular Epidemiology Resource (MER) and Lymphoma Epidemiology of Outcomes (LEO). Descriptive statistics were used to characterize patients included and excluded by various eligibility criteria. Chi-square tests and two-sample t-tests were used to compare categorical and continuous variables, respectively. To study the relative impact of individual criteria, we used a step-wise approach to quantify the number of additional patients excluded with each individual criterion after applying other criteria.
Results: Eligibility criteria included in at least one-third of studies included stage (present in all 21 trials), renal function (18), HIV/AIDS status (17), performance status (16), history of other malignancies (16), hepatic function (16), and cardiac function (15), other serious health conditions (13), pregnancy status (10), neuro/psych function (10), age (10), metabolic disease status (9), CNS involvement (9), birth control (8), pulmonary function (8), WBC count (8), HBV status (8), platelet count (7), measurable disease (7), HCV status (7), active infection status (7), and CD20 status (7).
We included 738 patients with newly diagnosed FL undergoing first-line therapy from MER and 703 patients from LEO. In MER, the median age was 60 years; 87% were White vs 2% non-White, 80% were non-Hispanic vs 2% Hispanic, and 68% were stage III/IV. In LEO, the median age was 60 years; 88% were White vs 11% non-White, 87% were non-Hispanic vs 11% Hispanic, and 67% were stage III/IV.
Exclusion criteria impacting more than 10% of patients included stage (31 in LEO vs 29% in MER), self-reported serious health conditions (17 vs 31%), prior cancer diagnosis (14 vs 11%), performance status (10%), and platelet count (10%). Patients excluded due to the following criteria tended to be older: renal function (median 70 vs 60 years in MER), prior malignancy (67 vs 58 years), and self-reported serious health conditions (66 vs 58 years). This pattern was consistent in LEO (Table). No eligibility criteria significantly impacted race/ethnicity.
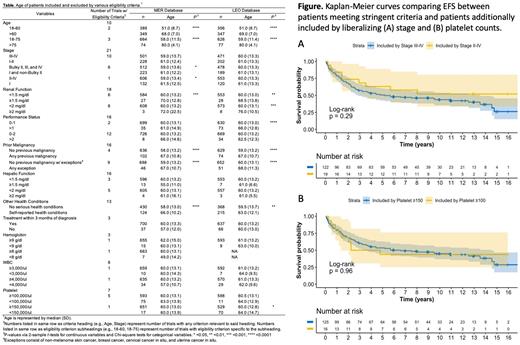
Given the potential for multiple eligibility criteria to exclude the same patients, we used a step-wise approach to evaluate the impact of individual criteria on patients remaining after exclusion by the larger group of criteria, and whether liberalizing criteria impacted patient numbers and/or demographics. We found that only self-reported serious health conditions significantly impacted numbers (excluding 43% of patients), whereas hematologic parameters and performance status excluded only 5 and 1% of patients, respectively, with no impact on age or race/ethnicity. We also found that liberalizing stage requirement from III-IV to II-IV could increase enrollment by 14% in MER (12% in LEO), and liberalizing platelet requirement from ≥150,000 to ≥100,000 could increase enrollment by 11% (15% in LEO). Moreover, liberalizing eligibility criteria had no impact on age or race/ethnicity of the patient pool or on EFS (Figure).
Conclusions: We found that excluding patients with prior malignancies, poor renal function, and self-reported serious health conditions appears to inadvertently exclude older patients. Other serious health conditions appeared to exclude a significant number of patients but did not impact patient demographics. Liberalizing certain criteria, including stage and platelet requirement, could potentially increase trial accrual, but would not likely correct the older patient deficit. These findings may serve in developing consensus eligibility criteria for future first-line FL trials.
Flowers: Bayer: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; National Cancer Institute: Research Funding; Allogene: Research Funding; Biopharma: Consultancy; Cellectis: Research Funding; EMD: Research Funding; Genentech/Roche: Consultancy, Research Funding; Epizyme, Inc.: Consultancy; Genmab: Consultancy; Iovance: Research Funding; Guardant: Research Funding; Burroughs Wellcome Fund: Research Funding; Sanofi: Research Funding; Takeda: Research Funding; Spectrum: Consultancy; Gilead: Consultancy, Research Funding; Amgen: Research Funding; Xencor: Research Funding; Nektar: Research Funding; SeaGen: Consultancy; Ziopharm: Research Funding; TG Therapeutics: Research Funding; 4D: Research Funding; Acerta: Research Funding; Denovo: Consultancy; Novartis: Research Funding; Pfizer: Research Funding; Eastern Cooperative Oncology Group: Research Funding; Morphosys: Research Funding; Adaptimmune: Research Funding; Pharmacyclics/Janssen: Consultancy; Karyopharm: Consultancy; BeiGene: Consultancy; AbbVie: Consultancy, Research Funding; Kite: Research Funding; Janssen: Research Funding; Cancer Prevention and Research Institute of Texas: CPRIT Scholar in Cancer Research: Research Funding; Pharmacyclics: Research Funding. Link: MEI: Consultancy; Novartis, Jannsen: Research Funding; Genentech/Roche: Consultancy, Research Funding. Friedberg: Acerta: Other: DSMC ; Bayer: Other: DSMC ; Novartis: Other: DSMC . Cohen: Genentech, Takeda, BMS/Celgene, BioInvent, LAM, Astra Zeneca, Novartis, Loxo/Lilly: Research Funding; Janssen, Adaptive, Aptitude Health, BeiGene, Cellectar, Adicet, Loxo/Lilly, AStra ZenecaKite/Gilead: Consultancy. Kahl: AbbVie, Acerta, ADCT, AstraZeneca, BeiGene, Genentech: Research Funding; AbbVie, Adaptive, ADCT, AstraZeneca, Bayer, BeiGene, Bristol-Myers Squibb, Celgene, Genentech, Incyte, Janssen, Karyopharm, Kite, MEI, Pharmacyclics, Roche, TG Therapeutics, and Teva: Consultancy. Lossos: Lymphoma Research Foundation: Membership on an entity's Board of Directors or advisory committees; Stanford University: Patents & Royalties; Seattle Genetics: Consultancy; NCI: Research Funding; Verastem: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; NIH grants: Research Funding; University of Miami: Current Employment. Nastoupil: Bayer: Honoraria; Gilead/Kite: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; ADC Therapeutics: Honoraria; IGM Biosciences: Research Funding; Takeda: Honoraria, Other: DSMC, Research Funding; Janssen: Honoraria, Research Funding; Genentech: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; TG Therapeutics: Honoraria, Research Funding; Epizyme: Honoraria, Research Funding; Denovo Pharma: Other: DSMC; Caribou Biosciences: Research Funding; Bristol Myers Squibb/Celgene: Honoraria, Research Funding; MorphoSys: Honoraria. Maurer: Nanostring: Research Funding; Pfizer: Membership on an entity's Board of Directors or advisory committees; Kite Pharma: Membership on an entity's Board of Directors or advisory committees; Morphosys: Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Research Funding; BMS: Research Funding. Cerhan: NanoString: Research Funding; Celgene/BMS: Other: Connect Lymphoma Scientific Steering Committee, Research Funding; Regeneron Genetics Center: Other: Research Collaboration; Genentech: Research Funding. Martin: ADCT: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal