Abstract
BACKGROUND
The revised genetic risk classification established by the European Leukemia Net (ELN) in 2017 stratifies patients diagnosed with acute myeloid leukemia (AML) into 3 prognostic categories (favourable, intermediate, and adverse) based on cytogenetic and molecular characteristics.The ELN classification is widely accepted in AML patients despite the fact that validation studies were performed in participants who received exclusively first-line treatment with intensive chemotherapy. For this reason, it is not well established whether the ELN risk groups are applicable to patients on non-intensive first-line treatment.
OBJECTIVES
- To describe and compare baseline characteristics at diagnosis between patients with AML treated with intensive and non-intensive therapy.
- To assess whether the ELN prognostic classification is applicable in these subgroups of patients.
METHODS
We retrospectively analysed patients with newly diagnosed AML admitted to our center between 2007 and 2020. Patients with acute promyelocytic leukemia (M3), patients younger than 18 years old and/or patients who received exclusively supportive treatment were excluded. Demographic and clinical data, disease characteristics at diagnosis and first-line treatment were collected. Cytogenetic and molecular characteristics were used to classify patients in ELN risk groups.
RESULTS
Of the total of patients (n=218), one hundred and fifty-six (71.6%) received intensive chemotherapy treatment, while 62 (28.4%) were treated with non-intensive strategies. Idarubicin and cytarabine based schemes regimens (IA) were administered in most patients (98.6%) who received intensive treatment while the rest received fludarabine based regimens. One patient (0.6%) was treated with danurubicin and cytarabine liposome (CPX-351). Fifty-four (87%) patients treated with non-intensive regimens received hypomethylating agents, mostly azacitidine. Five patients (8%) were treated with venetoclax in combination with a hypomethylating agent.
Table 1 shows the characteristics at diagnosis in both groups of patients. Patients who received intensive chemotherapy were younger and had higher leukocyte count, LDH values and a higher percentage of blasts in peripheral blood and bone marrow with a median of 40% and 62% blasts respectively. On the other hand, patients under non-intensive treatment more frequently presented a past history of hemopathy and a higher percentage of bone marrow dysplasia.
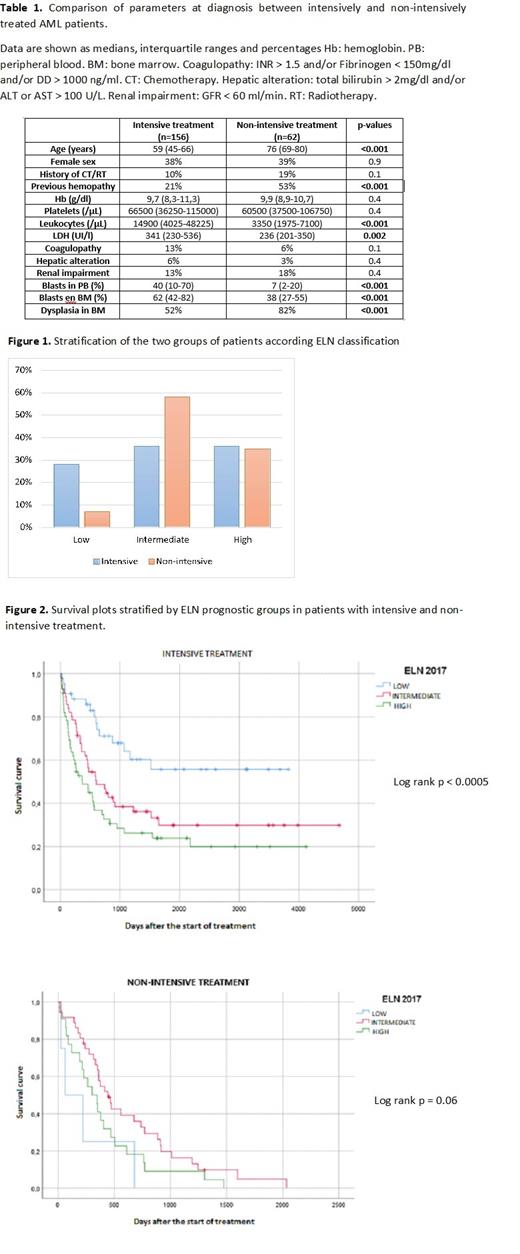
Regarding ELN stratification significant differences were found between both groups. Patients who received aggressive chemotherapy vs patients who did not, were classified in low (28% vs. 7%), intermediate (36% vs. 58%) and high risk (36% vs. 35%) respectively (Figure 1).
At the end of the follow-up, 41% of the patients who had received intensive therapy were alive while only 6.5% of the patients who had received non-intensive treatment were alive. Significant differences in survival were observed between both groups (p<0.01); with 1-year overall survival (OS) of 65.8% for intensive therapy group and 49.6% for non-intensive therapy group.
In the intensive chemotherapy group, significant differences in survival were observed according to ELN risk stratification (p<0.01), with 5-year OS of 55%, 29% and 23.9% for low, intermediate and high-risk groups respectively. For low-risk patients, median OS was not reached while it was 20 months for the intermediate risk group and 12.2 months for the high-risk group.
However, in patients receiving non-intensive therapies, there were no significant differences in survival among different prognostic categories (p=0.06). In this group, 1-year OS was 25%, 57.6% and 40.7% and median OS was 2.1, 14.8 and 10.1 months for low, intermediate and high-risk groups respectively. See Figure 2.
CONCLUSIONS:
As validated in previous trials, ELN classification constitutes an adequate prognostic marker for patients with newly diagnosed AML treated with intensive chemotherapy. In our series, this classification does not appear to be a good predictor of survival for patients diagnosed with AML who initiated non-intensive treatments. Further validation in prospective studies are needed to better classify this growing subgroup of patients in clinical practice.
Martín-Rojas: Celgene-BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees. Font Lopez: Pfizer: Membership on an entity's Board of Directors or advisory committees; GILEAD: Membership on an entity's Board of Directors or advisory committees; CELGENE-BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees. Kwon: Novartis, Celgene, Gilead, Pfizer: Consultancy, Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal