Abstract
Introduction
Polycythemia Vera (PV) is a chronic myeloproliferative neoplasm that presents with increase proliferation of red cells as well as variable presence of thrombocytosis & leukocytosis. Currently treatment options for PV are phlebotomy, low-dose aspirin or cytoreductive therapy. Interferon (IFN) is a biological response modifier that exerts myelosuppressive action on excessively proliferative cell lineages and is also a non-leukemogenic drug. We conducted a systematic review and meta-analysis on the efficacy of Interferon for the treatment of PV.
Methods
Following the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines, a comprehensive literature search was conducted on PubMed, Cochrane, and Clinical trials.gov using MeSH terms and keywords for " Polycythemia Vera " AND " Interferons ". A total of 577 records were discovered using database searching. All search results were imported into the Endnote X9.0 reference manager, and duplicates were removed. After screening and excluding review and irrelevant articles, 22 original articles reporting IFN as treatment for PV in adult patients were included. The data were collected for baseline characteristics of the participants and efficacy and safety of the intervention. Quality evaluation was done using the NIH quality assessment tool. The inter-study variance was calculated using the Der Simonian-Laird Estimator. Proportions along with 95% Confidence Interval (CI) were extracted to compute pooled analysis using the 'meta' package by Schwarzer et al. in the R programming language (version 4.16-2).
Results
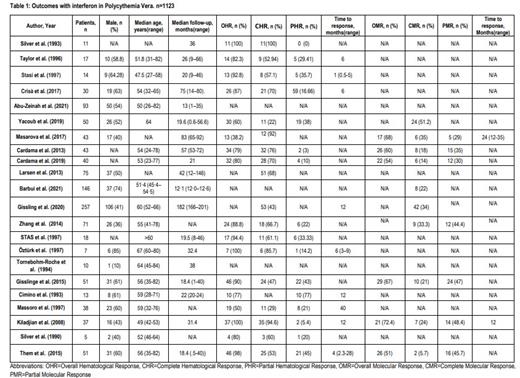
A total of 1123 patients were evaluated from 22 studies. The median age was 54.5 (47.5-67) years and median follow-up time was 24 (9-146) months. The median prior number of phlebotomies was 4.55 (2-18). The type of IFN used were recombinant IFN-alpha-2a in 2 studies, IFN-a2b in 5 studies, recombinant IFN-a in 6 studies, and Pegylated-recombinant IFN-α2a in 7 studies. The pooled overall hematological response (OHR) was 86% (95% Cl 0.76-0.93, I 2= 84%, p=<0.01, n=460) with pooled complete hematological response (CHR) of 63% (95% Cl 0.50-0.76, I 2=85%, p=<0.01, n=409) and pooled partial hematological response (PHR) of 22% (95% Cl 0.12-0.34, I 2=81%, p=<0.01, n=361). Pooled overall molecular response (OMR) was 64% (95% Cl 0.56-0.71, I 2=0%, p=0.6, n=190) with pooled complete molecular response (CMR) of 24% (95% Cl 0.14-0.35, I 2=75%, p=<0.01, n=276) and pooled partial molecular response (PMR) of 38% (95% Cl 0.31-0.45, I 2=0%, p=0.5, n=191). Side effects reported were nausea, allergic reactions, liver dysfunction, dose dependent mild myalgia, fever, malaise, itching, persistent fever, headache, and flu like symptoms [Table 1].
Conclusion
Interferon shows promising results when used for the treatment of polycythemia vera with a durable hematologic and molecular response and has an acceptable side effects profile. However, large randomized clinical trials are needed to confirm these findings and to explore the dose and combination of interferon with other drugs..
Abhyankar: Incyte/Therakos: Consultancy, Research Funding, Speakers Bureau. McGuirk: Bellicum Pharmaceuticals: Research Funding; Juno Therapeutics: Consultancy, Honoraria, Research Funding; Pluristem Therapeutics: Research Funding; Novartis: Research Funding; Allovir: Consultancy, Honoraria, Research Funding; EcoR1 Capital: Consultancy; Novartis: Research Funding; Magenta Therapeutics: Consultancy, Honoraria, Research Funding; Fresenius Biotech: Research Funding; Kite/ Gilead: Consultancy, Honoraria, Other: travel accommodations, expense, Kite a Gilead company, Research Funding, Speakers Bureau; Astelllas Pharma: Research Funding; Gamida Cell: Research Funding. Yacoub: Dynavex: Current equity holder in publicly-traded company; Cara: Current equity holder in publicly-traded company; Ardelyx: Current equity holder in publicly-traded company; Agios: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ACCELERON PHARMA: Membership on an entity's Board of Directors or advisory committees; CTI Biopharma: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Consultancy, Honoraria, Speakers Bureau; Seattle Genetics: Honoraria, Speakers Bureau; Hylapharm: Current equity holder in publicly-traded company.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal