Abstract
Introduction
CD19-targeted chimeric antigen receptor (CAR)-T cell therapy effective for refractory/relapsed (R/R) B-cell acute lymphoblastic leukemia (B-ALL) that results in about 90% of complete remission (CR)/CR with incomplete blood recovery (CRi). According to a published study from our center that analyzed 254 R/R B-ALL patients, we confirmed that TP53 mutation was an independent prognostic factor of efficacy following CD19 CAR-T therapy. What factors affect the prognosis of patients with TP53 mutation/deletion treated with CD19 CAR-T is not clear. Here, we focus on the patients with TP53 mutation/deletion and analyze the factors associated with efficacy following CD19 CAR-T therapy among 64 B-ALL patients with TP53 mutation/chromosome 17p deletion.
Patients and Methods
From June 2017 to February 2020, we analyzed 64 R/R patients (36 male, 28 female) with TP53 mutation/chromosome 17p deletion who received CD19 CAR T-cells from 5 clinical trials at Hebei Yanda Lu Daopei Hospital (NCT03173417, NCT02546739, NCT03312205 and NCT03671460 registered at https://clinicaltrials.gov; Chictr-onc-17012829 at www.chictr.org.cn). Among them, there were 42 patients with TP53 mutation only, 10 with chromosome 17p deletion, and 12 harboring both the mutation and deletion. There were 27 pediatric patients ≤ 14 years old and the remaining 37 patients were adults (>14 years old).
Results
After CAR-T therapy, 49/64 (76.6%) patients achieved CR/CRi on Day 30 and 1-year OS among the 64 patients was 39.4%. Other 15 patients who had no response (NR) to CAR-T therapy died within 1 year. Among the 49 patients who achieved CR after CD19 CAR-T, 1-year OS and 1-year relapse-free survival (RFS) were 50.0% and 40.9%, respectively. Thirty-three of the 49 patients subsequently bridged to allogeneic hematopoietic stem cell transplantation (allo-HSCT) with 1-year OS and RFS of 59.5% and 55.8%, respectively.
Using univariate analysis of CR rates (Table 1), the pediatric patients demonstrated a lower CR rate compared to the adult patients (63.0% vs. 86.5%, p=0.04). We saw a trend of patients with complex cytogenetics having a relatively low CR rate compared to patients without complex cytogenetics yet no significant difference was observed (69.4% vs.85.7%, p= 0.15). For the TP53 functional mutation group versus the TP53 non-functional mutation group, CR rates were 70.0% vs. 92.8%, respectively (p= 0.15). In addition, patients with more than one TP53 mutations (≥ 2) showed a lower CR rate of 72.3% compared to patients with one TP53 mutation who achieved 100% CR (p= 0.18).
Analyzing the data from all 64 patients, we identified three factors that were significantly associated with OS: 1) presence of complex cytogenetics (compared to without complex cytogenetics; p= 0.005), 2) achievement of CR/CRi (compared to NR; p< 0.001), and 3) bridging into allo-HSCT post CAR-T (compared to CAR-T only; p < 0.001).
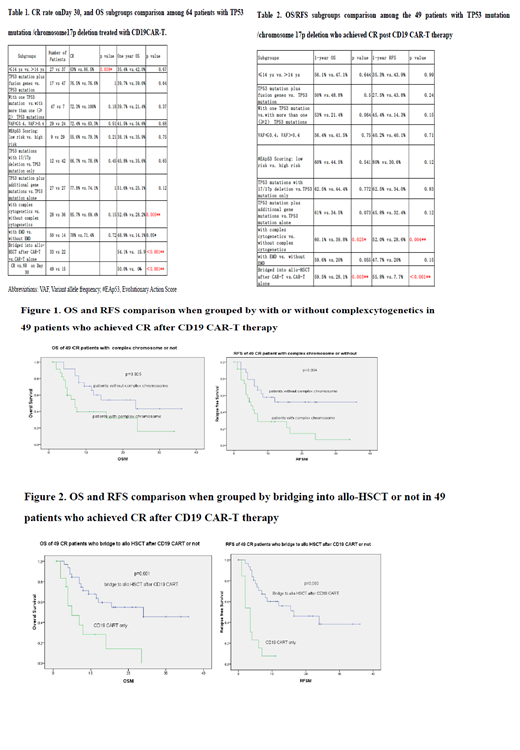
Next, we focused on analyzing the factors affecting the long-term efficacy of the 49 patients who achieved CR (Table 2). Two factors were associated with OS/RFS: 1) presence of complex cytogenetics (compared to no complex cytogenetics; 1-year OS of 60.1% vs. 39.8%, p=0.025; 1-year RFS 52.0% vs. 28.6%, p = 0.004, Figure 1), and 2) bridging into allo-HSCT post CAR-T (compared to CAR-T only; 1-year OS 59.5% vs. 28.1%, p = 0.003; 1-year RFS 55.8% vs. 7.7%, P < 0.001, Figure 2). Four additional factors that showed a trend of worse efficacy were 1) TP53 mutations more than one (≥2) (compared to one TP53 mutation; 1-year 53% vs. 21.4%, p=0.06; 1-year RFS 45.4% vs. 14.3%, p=0.15), 2) presence of TP53 mutations plus additional gene mutations (compared to TP53 mutation alone; 61% vs. 34.5%, p=0.07), 3) patients with extramedullary disease (EMD) (compared to without EMD; 1-year OS 59.6% vs. 20%, p= 0.05; 1-year RFS 47.7% vs. 20%, p=0.15), and 4) low risk evolutionary action score (EAp53) (compared to high risk; 1-year RFS 80% vs. 30.6%, p=0.12).
Conclusions
This study has uncovered factors that may predict treatment outcomes and duration of remission for patients with TP53 mutation/chromosome 17p deletion who received CD19 CAR-T therapy. Presence of complex cytogenetics and not bridging into allo-HSCT are two factors affecting the long-term efficacy. Further studies are needed to confirm these results, to improve the clinical management and personalization of treatment approaches for B-ALL patients harboring TP53 mutation/chromosome 17p deletion.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal