Abstract
Introduction
CAR T-cell therapies are utilized to treat relapsed/refractory B-lymphoblastic leukemia (B-ALL), diffuse large B cell lymphoma, mantle cell lymphoma, and multiple myeloma. CAR T cell expansion kinetics after infusion impact response. However, these CAR T cell products vary in their target epitope and constituent molecules that makes it difficult to validate broad molecular or flow cytometric assays for use in the clinical setting. The lack of commercially available reagents also limits the ability of clinical laboratories to validate separate CAR T cell specific assays. We investigated the utility of common hematology laboratory parameters to measure CAR T cell expansion and response.
Methods
Clinical hematology laboratory parameters after infusion of autologous and allogeneic CAR T cell products directed were assessed. Concurrent CBC and cell population data (CPD) parameters from the Sysmex XN 3000 automated hematology analyzer were available in 82 patients. Absolute lymphocyte count (ALC) kinetics after infusion of autologous CD19, allogeneic CD19, CD22, CD33, allogeneic CD123-directed CAR T cell products were analyzed. Patients who received bone marrow transplant served as controls. CPD parameters included X (lateral light scatter-granularity), Y (fluorescence-nuclei acid content), Z (forward scatter-size), and their distribution widths WX, WY, WZ. Archived CellaVision cell morphology images from 118 patients who received CD19-directed CAR T cell products and 25 patients who received other CAR T cell products were analyzed. Response was determined from 1 month post-CAR bone marrow disease assessment.
Results
Absolute Lymphocyte Counts, lymphocyte morphology and cell population data after infusion of CAR T cell products
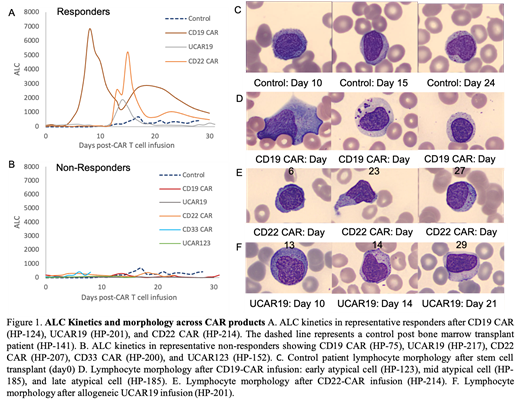
CD19-CAR, UCAR19, and CD22-CAR all showed a distinct lymphocyte expansion phase post-infusion in responders (Figure 1A) that was absent in non-responders and controls(Figure 1B). ALC showed characteristic lag, expansion, and contraction phases in responders. CD19-CAR had a peak at day 8 while CD22-CAR and allogenic Universal(U)CAR19 had relatively delayed peak times occurring near day 15 (Figure 1B). The stem cell transplant control patients did not show ALC expansion in the first two weeks and instead showed normal lymphocyte regeneration that occurs in the third to fourth weeks. CD33-CAR and allogeneic UCAR123 non responders did not show ALC expansions.
CAR T cell responders showed a distinct sequence of changes in lymphocyte morphology that was absent in non-responders and stem cell transplant controls (Figure 1C). This pattern was noted uniformly across various CAR T cell products (Figure 1D-1F). The morphological changes were categorized as: early, mid, and late. Early atypical cells were noted around days 4-8 after infusion and showed immunoblastic morphology that was present in 89% (n=105) of patients. Mid atypical cells were noted around days 5-14 after infusion and showed atypical large granular lymphocyte morphology that were seen in 95% (n=112) of patients. Late atypical cells showed the typical LGL morphology and was seen in 82% (n=97) of patients.
WY fluorescence, which is a measure of nucleic acid content, was most useful in assessing changes in lymphocytes after CAR T cell infusion. WY was low (mean 402, n=57) at baseline pre-infusion timepoint and peaked (mean 1207) approximately 1 week after infusion (mean 7.9 days, n = 59). Peak WY was observed 3.7 days prior to peak ALC (mean day 11.6, n = 59).
Conclusion
We demonstrate for the first time that common clinical laboratory parameters can be used to follow CAR T cell expansion after infusion in various autologous and allogeneic CAR T cell products. ALC expansion is a measure of CAR T cell expansion after infusion and correlates with response. Responders showed higher peak ALC compared to non-responders in all CAR T cell products. Timing of peak ALC expansion was determined by other factors such as expression of target antigen and CAR T-cell product used. Lymphocyte morphology followed ALC changes and showed a consistent sequence of changes that was seen across multiple CAR T cell products. Finally, CPD parameter WY which is a measure of nuclei acid content and activation, was an early harbinger of ALC expansion.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal