Abstract
Background:
Bispecific anti-CD20, anti-CD19 (LV20.19) CAR T-cells may improve outcomes in relapsed, refractory (R/R) non-Hodgkin lymphoma (NHL) by limiting relapse due to single antigen downregulation. We recently reported outcomes of a phase I trial of LV20.19 CAR T-cells expanded in IL-2 in R/R NHL & CLL (Shah Nat Med 2020). Preclinical models demonstrated CAR T-cells expanded in IL-7 & IL-15 (IL7+15) had improved persistence and anti-tumor efficacy compared to IL-2 (Xu Blood 2014). It is unknown how the length of manufacturing modulates the composition and efficacy of the CAR-T product in the clinical setting. To optimize our CAR-T product, we opened a phase I/II trial of LV20.19 CAR T-cells expanded in IL-7+15 manufactured under variable lengths of time (8 vs 12 days) in R/R NHL (NCT04186520).
We describe manufacturing and immunophenotypic differences for LV20.19 CAR T-cells expanded in IL7+15 and manufactured for 8- or 12-days versus (vs) cells expanded in IL-2 and manufactured for a fixed 14-days.
Methods:
LV20.19 CAR T-cells were manufactured onsite with the CliniMACS Prodigy device. Immunophenotyping was conducted for CAR-T products using 8-color flow cytometry. A panel of markers to assess T-cell differentiation status and an immune checkpoint protein panel (LAG3, TIM3, & PD1) were used to analyze IL7+15 expanded CAR T-cells. Testing during the manufacturing process was done to determine: %CD3 T-cells, CD4:CD8 ratios (total T cells & CAR T-cells), #CD3 T-cells, fold CD3 T-cell expansion, %CAR+ T-cells, #CAR T-cells, and % viability at days 0, 7 & 8 or day 12 for IL7+15 products, and days 0, 8 & 14 for IL-2 products. Variables were analyzed with parametric or non-parametric tests where appropriate. Analysis was limited to those treated at 2.5x10 6 cells/kg. A p-value <.05 was considered significant. All p-values are 2-tailed.
Results:
There were 21 patients from the IL7+15 LV20.19 trial and 12 from the LV20.19 IL-2 trial included in the analyses. In the IL7+15 trial, there were 13 patients included in the 8-day manufacturing arm and 8 in the 12-day arm.
Manufacturing differences of products expanded in IL7+15 vs IL-2
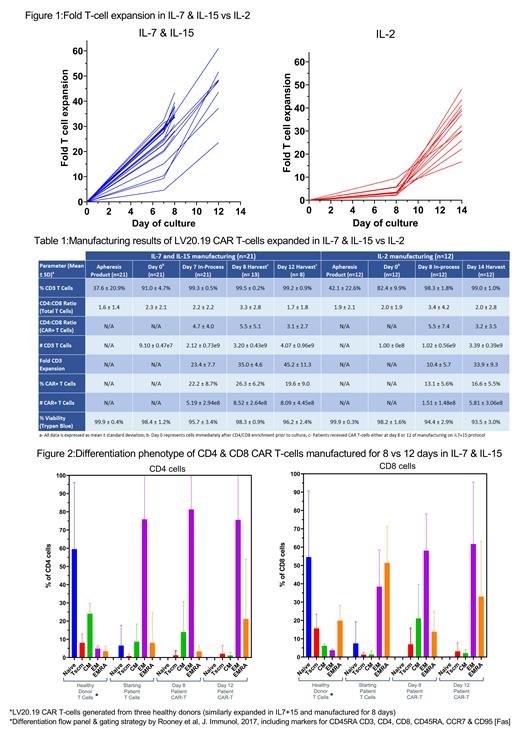
LV20.19 CAR T-cells expanded in IL7+15 had over 2 times greater fold CD3 expansion by day 7 than IL-2 expanded cells at day 8 (23.4± 7.7 vs 10.4 ± 5.7) (figure 1). Fold CD3 expansion was also higher on day 12 (n=8) for IL7+15 expanded cells at 45.2 ± 11.3 vs 33.9 ± 9.3 on day 14 for IL-2 expanded cells. Although starting numbers of CD3 T-cells were similar between arms, the number of CAR T-cells at day 7 was over 3-fold higher for IL7+15 vs IL-2 expanded cells at day 8 (5.19 ± 2.94e8 vs 1.51 ± 1.48e8)(table 1).
Immunophenotypic differences of products expanded in IL7+15 manufactured for 8 vs 12 days
LV20.19 CARs harvested at day 8 had a higher frequency of CAR T-cells with a central-memory (CM) phenotype (CD4 cells: 14.0% vs 1.1%, p=0.001; CD8 cells: 21.1% vs 2.1%, p=0.009) (figure 2). The 8-day arm had a lower frequency of CAR T-cells with a terminally differentiated effector-memory CD45RA+ (EMRA) phenotype (CD4 cells: 21.2% vs 3.4%, p=0.06; CD8 cells: 33.0% vs 13.9%, p=0.003). There were no significant differences in the frequency of naïve, T-stem cell memory (Tscm), or effector memory (EM) phenotypes between arms. However, T-cells with a naïve phenotype were virtually absent from the day 12 products but still present in day 8 products. Expression of immune checkpoint proteins was similar between 8- and 12-day arms.
Conclusions:
Manufacturing LV20.19 CAR T-cells with IL7+15 leads to improved CD3 fold expansion and greater absolute numbers of CAR T-cells vs IL-2. IL7+15-expanded LV20.19 CAR T-cells manufactured for a shorter duration (8 days) have a higher frequency of cells with a CM phenotype and less terminally differentiated cells than those manufactured for 12 days. Central-memory T-cells have been found to have superior in vivo persistence than EM T-cells (Berger, J Clin Invest 2008), and CAR-T products generated from CM T-cells have greater antitumor efficacy than those from EM T-cells (Sommermeyer, Leukemia 2016). Given the composition of CAR-T products has been shown to impact anti-tumor efficacy, this suggests that manufacturing bispecific CAR T-cells in IL7+15 for a shorter duration could lead to greater clinical efficacy without negatively impacting attainment of cell dose. Clinical outcomes of 8- vs 12-day manufactured IL-7+15 expanded LV20.19 CAR T-cells will be reported separately.
Schneider: Employee of Lentigen Technology, a Miltenyi Biotec Company: Current Employment. Hari: Oncopeptides: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Adaptive Biotech: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding, Speakers Bureau; Millenium: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding, Speakers Bureau; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding, Speakers Bureau; Karyopharm: Consultancy; GSK: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding, Speakers Bureau; Celgene-BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding, Speakers Bureau; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau. Johnson: Miltenyi Biotec: Research Funding. Shah: Umoja: Consultancy; Miltenyi Biotec: Consultancy, Honoraria, Research Funding; Kite: Consultancy; Incyte: Consultancy; Epizyme: Consultancy; Legend: Consultancy; Lily: Consultancy, Honoraria, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal