Abstract
Background: Patients with acute myeloid leukemia (AML) who receive intensive chemotherapy must cope with immense physical and psychological symptoms associated with a variety of patient-reported outcomes (PROs) such as quality of life (QOL). Although coping is critical to the management of an AML diagnosis and its treatment, data characterizing the use of coping strategies and its associations with PROs in the AML population are limited. Hence, we characterize coping strategy use among patients with AML and examine the associations between coping strategy use, psychological distress, and QOL.
Methods: We used cross-sectional secondary data analyses to describe coping in 160 patients with newly diagnosed high-risk AML enrolled in a multi-site randomized supportive care trial. We used the Brief COPE, Hospital Anxiety and Depression Scale (HADS), PTSD Checklist-Civilian Version (PCL-C), and Functional Assessment of Cancer Therapy-Leukemia (FACT-Leu) within 72 hours of patient initiation of chemotherapy, to measure coping strategies, psychological distress and QOL, respectively. We grouped coping strategies into two higher-order domains of coping based on prior literature: approach-oriented coping (i.e., use of emotional support, active coping, positive reframing, acceptance) or avoidant coping (i.e., self-blame, denial, behavioral disengagement). We used the median split method for the distribution of coping domains. We used multivariate regression models adjusting for age, gender and diagnosis type (newly diagnosed vs. relapsed/refractory AML) to assess the relationship between coping and PROs.
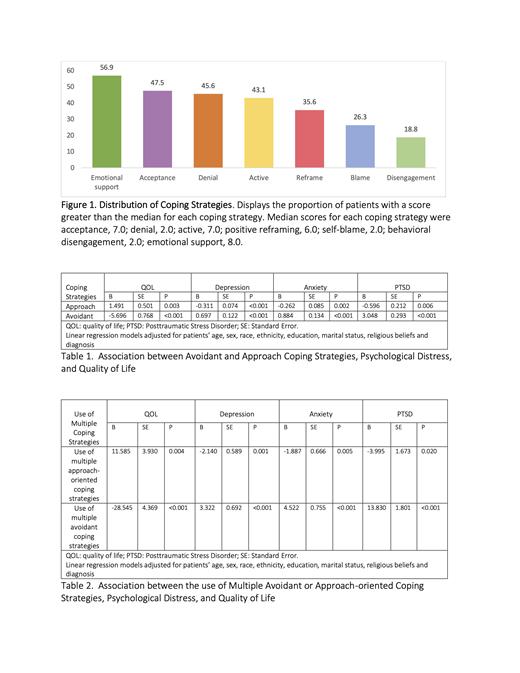
Results: Participants (median age of 64.4 years) were mostly non-Hispanic White (86.3%), male (60.0%), and married (73.8%). Most (51.9%) reported high utilization of approach-oriented coping strategies (e.g., emotional support) whereas 38.8% reported high utilization of avoidant coping strategies (e.g., denial) (Figure 1). At the time of AML diagnosis, use of approach-oriented coping was associated with less psychological distress and better QOL (Table 1). Use of avoidant coping was associated with more psychological distress and worse QOL. Additionally, patients who used multiple approach-oriented coping strategies had less psychological distress and better QOL (Table 2). In contrast, patients who used multiple avoidant coping strategies had more psychological distress, and worse QOL.
Conclusions: Our study illustrates that most patients with high-risk AML utilize both approach-oriented and avoidant coping strategies. Our results also reveal links between approach-oriented coping strategies, less psychological distress, and better QOL. These findings underscore the need for early integration of supportive oncology interventions that help patients to cultivate approach-oriented coping strategies.
Brunner: Novartis: Consultancy, Research Funding; BMS/Celgene: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Acceleron: Consultancy; Agios: Consultancy; Keros Therapeutics: Consultancy; GSK: Research Funding; Aprea: Research Funding; AstraZeneca: Research Funding; Janssen: Research Funding. Fathi: AbbVie: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Blueprint: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria; Astellas: Consultancy, Honoraria; Daiichi Sankyo: Consultancy, Honoraria; Genentech: Consultancy, Honoraria; Trillium: Consultancy, Honoraria; Kura: Consultancy, Honoraria; Foghorn: Consultancy, Honoraria; Kite: Consultancy, Honoraria; Morphosys: Consultancy, Honoraria; Ipsen: Consultancy, Honoraria; Agios: Consultancy, Honoraria, Research Funding; Servier: Research Funding; Celgene/BMS: Consultancy, Honoraria, Research Funding. LeBlanc: Seattle Genetics: Consultancy, Other: Advisory board, Research Funding; Pfizer: Consultancy, Other: Advisory Board; AstraZeneca: Consultancy, Honoraria, Other: Advisory board, Research Funding; UpToDate: Patents & Royalties; American Cancer Society: Research Funding; Agios: Consultancy, Honoraria, Other: Advisory board; Travel fees, Speakers Bureau; BMS/Celgene: Consultancy, Honoraria, Other: Travel fees, Research Funding, Speakers Bureau; Daiichi-Sankyo: Consultancy, Honoraria, Other: Advisory board; Flatiron: Consultancy, Other: Advisory board; Astellas: Consultancy, Honoraria, Other: Advisory board; AbbVie: Consultancy, Honoraria, Other: Advisory board; Travel fees, Speakers Bureau; Otsuka: Consultancy, Honoraria, Other; Jazz Pharmaceuticals: Research Funding; Duke University: Research Funding; Helsinn: Consultancy, Research Funding; Heron: Consultancy, Honoraria, Other: advisory board; CareVive: Consultancy, Other, Research Funding; NINR/NIH: Research Funding; Amgen: Consultancy, Other: travel.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal