Abstract
Introduction: Acute myeloid leukemia (AML) is a disease of the older population, with a median age at diagnosis of 68 years. Outcomes remain poor for patients (pts) older than 60 years of age who are unfit to receive intensive chemotherapy. Ven based combination therapy has become the standard of care based on the results of the Viale-A and Viale-C in combination with hypomethylating agents (HMA) or low dose cytarabine (LDAC), respectively. This combination therapy is myelosuppressive leading to multiple dose adjustments in the real-world setting, which may impact the validity of the trial responses and overall survival (OS) in a community-based practice. We therefore sought to investigate the outcomes of pts treated with ven based combination therapy in the real-world setting to better understand efficacy and patterns of adverse events.
Methods: Pts 65 years of age or older, diagnosed with AML on or after January 1, 2019, and who received frontline ven based combination therapy with azacitidine (aza), decitabine (dac) or LDAC were identified in the COTA real-world database. The COTA database is a USA-based dataset comprised of longitudinal, Health Insurance Portability and Accountability Act (HIPAA)-compliant data on the diagnosis, clinical management, and outcomes of pts with cancer. Clinical outcomes, including real-world overall response rate (rwORR), OS and adverse events were calculated by treatment group. Responses were defined per treating physician as complete response (CR) or partial (PR) or progressive disease (PD). Categorical variables and rwORR were compared using Fisher's exact test. Survival outcomes were calculated using the Kaplan-Meier method and compared amongst groups using log-rank test.
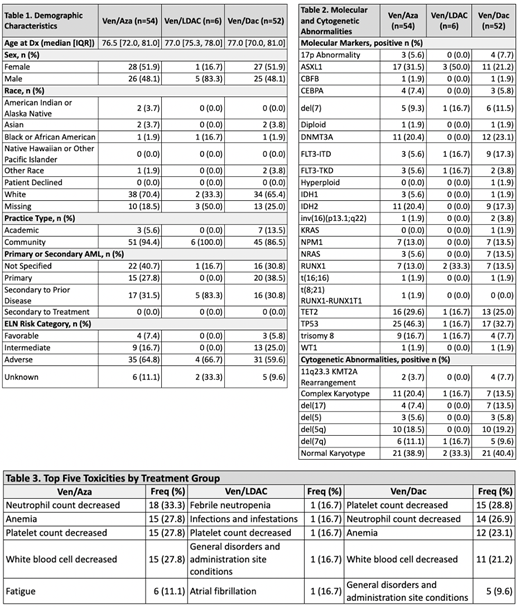
Results: A cohort of 112 pts were treated with ven based combination: ven/aza (n=54), ven/dac (n=52) and ven/LDAC (n=6). Across all treatment groups, the majority (91.1%) of pts were treated in the community setting. The median age at diagnosis was 77 yrs (IQR: 71-81 yrs). Per ELN risk, 6.2% had favorable, 19.6% had intermediate and 62.5% had high-risk AML. Five of the 6 pts receiving ven/LDAC had secondary AML (Table 1). The most common molecular abnormalities across the cohort were TP53 (38.4%), TET2 (26.8%), ASXL1 (27.7%), and DNMT3A (20.5%). The rwORR by treatment was 57.7% for ven/dac, 55.6% for ven/aza and 33.3% for ven/LDAC (ven/dac vs. ven/aza, p=0.85). Median OS (mOS) was similar for ven/dac with a longest mOS of 13.9 mo, followed by ven/aza with a mOS of 11.3 mo, and ven/LDAC mOS of 6.5 mo (ven/dac vs. ven/aza, p=0.77). The most common reasons for treatment discontinuation were toxicity (32.1%) followed by progression/inadequate response (10.7%). The most common toxicities were related to myelosuppression/cytopenias (Table 3). In this cohort, only 6 pts underwent an allogeneic stem cell transplant in first remission and no pts underwent an allogeneic transplant in second remission.
Conclusions: To our knowledge, this dataset represents the largest cohort of pts treated with ven based combination therapy in the real world setting across multiple community practices. As less intense regimens are used to treat AML, more pts are receiving induction in the community setting, making these findings relevant in clinical practice. Although ven/dac combination had the longest OS, this was not significantly different from ven/aza. The ven/HMA response rates were lower and survival outcomes were shorter than those reported in the Viale-A trial, which raises concerns about early discontinuation of therapy in the real world setting due to myelosuppression. Ven/LDAC combination had the shortest OS, though most of these patients had sAML. Despite this enriched population for targeted therapies, the outcomes remained poor overall.
There are drawbacks to interpreting retrospective outcomes data. First, there were no treatment related AML cases in this dataset, which would be highly unusual in an elderly population of 112 cases of AML. It is more plausible that the diagnosis of treatment related AML was not utilized in the patient's chart. Other data points that were not adequately captured were cause of death and International Working Group response criteria. Mutational panel testing remains underutilized for AML patients in a community-based practice setting. However, this dataset highlights the need for larger datasets to compare the outcomes of AML therapy by practice setting.
Madanat: Blue Print Pharmaceutical: Honoraria; Stem line pharmaceutical: Honoraria; Onc Live: Honoraria; Geron Pharmaceutical: Consultancy. Belli: COTA, Inc.: Current Employment, Other: Equity ownership. Hansen: COTA, Inc.: Current Employment. Foss: COTA, Inc.: Current Employment. Schulte: COTA, Inc.: Current Employment. Wang: COTA, Inc.: Current Employment, Other: Equity ownership.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal