Abstract
Introduction:
Sickle Cell Disease (SCD) is a hemolytic disorder with an increased risk of venous thromboembolism (VTE). By the age of 40 years around 11-12% of sickle cell disease patients will have at least one episode of VTE. VTE among patients with SCD is associated with a two to four times increase in mortality compared to SCD patients without VTE. Nevertheless, the evidence guiding VTE management in SCD, specifically in terms of anticoagulant choice, is scarce. Therefore, we conducted a systematic review that evaluates the effectiveness and safety of direct oral anticoagulants (DOACs) in SCD with VTE.
Methods:
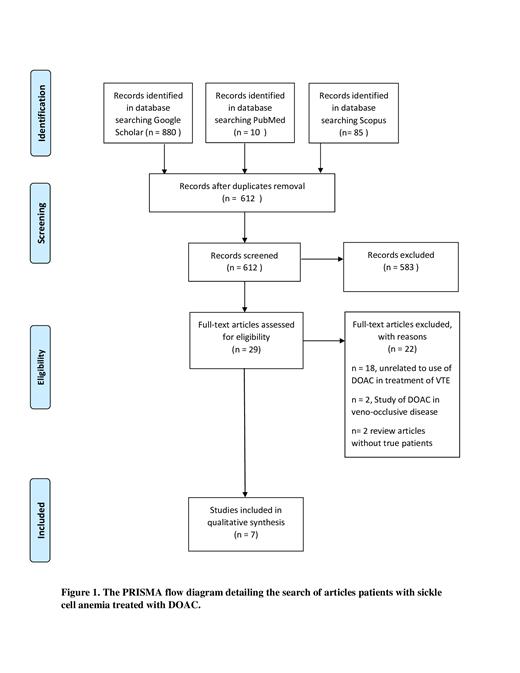
We performed a systematic review following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. We searched the English literature (PubMed, SCOPUS, and Google Scholar) for randomized controlled trials, observational studies, reviews, case series, and case reports for patients with SCD treated with DOAC for thromboembolic disease. We used the terms in combination: "Sickle cell disease" or "Sickle cell anemia", and "DOAC", "rivaroxaban", "apixaban", "dabigatran" "edoxaban". The search included all articles published up to 20th April 2021. Quality and risk of bias assessment were done by two independent authors for each included study.
Results:
A total of 7 articles were included; four observational studies, and three case series addressing this matter. Patel A et al. found that the use of DOACs, including rivaroxaban, dabigatran, and apixaban in comparison to vitamin K antagonists (VKAs) and low molecular weight heparin (LMWH) for the treatment of VTE in SCD among adults was associated with similar VTE recurrence rate and a better safety profile in terms of a significant reduction in major bleeding events. Similarly, Roberts MZ et al. reported that the use of DOACs for VTE treatment in SCD compared to VKAs resulted in similar effectiveness in terms of VTE recurrence, but the use of DOACs was associated with a similar safety in comparison to VKAs in contrary to the results reported by Patel A et al. in their retrospective study. With regards to the risk of major hemorrhagic events associated with the use of non-VKAs, Gupta VK et al. showed that among 55 patients with SCD treated with VKAs, DOACs, or injectable anticoagulants, only patients treated with VKAs had major bleeding events.
Discussion:
The current data demonstrated that the use of DOACs for VTE in SCD has similar effectiveness in the prevention of VTE recurrence in comparison to other anticoagulants, including VKAs and injectable anticoagulants with a better safety profile. However, given the absence of clinical practice guidelines for the treatment of VTE among patients with SCD, the clinical practice guidelines recommendations for VTE treatment can be applied to patients with SCD. According to the latest CHEST guidelines (2016) for the treatment of VTE, the use of DOACs is recommended in patients with VTE over VKAs. Similarly, the latest American Society of Hematology (2020) guidelines for VTE suggest the use of DOACs over VKAs, except among patients with renal insufficiency (creatinine clearance less than 30 mL/min), moderate to severe liver disease, or those with antiphospholipid syndrome.
Conclusion:
In view of the current evidence and based on the results observed; using DOACs was associated with lesser bleeding incidence and fewer complications comparing to VKAs. We think it is rational to use DOACs for VTE treatment among patients with SCD rather than use VKAs.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal