Abstract
Background
Penpulimab is an IgG1 anti-PD-1 antibody with Fc-engineering to eliminate effector functions. Treatment with penpulimab as a single agent at 1.0, 3.0 and 10.0 mg/kg Q2W cohort were safe during the phase Ia dose-escalation phase with no dose-limiting toxicity observed and 80-100% receptor occupancy being observed in all of the above dose cohorts. It was administered in a fixed dosing regimen [200 mg every 2 weeks (Q2W)] in a phase II study (AK105-201) demonstrating efficacy and safety in Relapsed/Refractory classic Hodgkin's lymphoma (R/R cHL). Longer dosing intervals may offer greater flexibility and convenience to both patients and healthcare professionals. To provide alternative extended dosing schedules for patients receiving penpulimab, we explored the feasibility of a longer dosing regimen [200 mg every 3weeks (Q3W) and 400 mg Q6W] using exposure-response (ER) analysis approach.
Methods
A penpulimab population PK(PopPK) model was established based on PK data from a total of 332 subjects from six clinical studies. The following PK parameters were determined and used for penpulimab E-R analysis: the trough concentrations at steady-state (C min,ss); the peak concentration at steady-state (C max,ss); area under the concentration-time curve at steady-state (AUC ss). ER evaluation using modeling and simulation was used to demonstrate the similarity of efficacy and safety for 200 mg Q3W and 400 mg Q6W regimens compared with 200 mg Q2W regimen. The efficacy endpoints used in the analysis were complete response (CR), overall response rate (ORR) and disease control rate (DCR) in AK105-201 study and the safety endpoints included ≥ grade 3 treatment-related adverse event (TRAE), ≥ grade 3 immune-related adverse events (irAE), or AE leading to suspension in all the six clinical studies.
Result
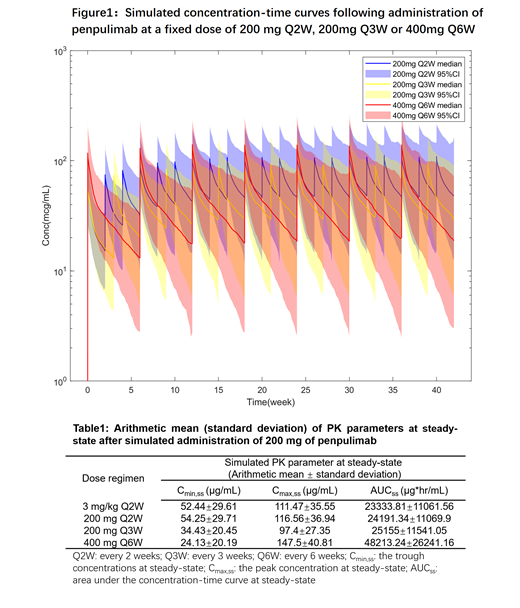
The final PopPK structural model for penpulimab was a two-compartment model with first-order elimination by intravenous infusion. The simulation showed that after multiple doses, the concentration-time curves for 200 mg Q2W or 3 mg/kg Q2w administration were generally consistent. Compared to the 200 mg Q2W regimen, the mean C min,ss of 200 mg Q3W and 400 mg Q6W regimen were approximately 36.5% and 55.5% lower, respectively. However, the fixed-dose simulation (200 mg Q3W, 400 mg Q6W) showed that the C min,ss of 97.5% of the population were higher than 3 μg/mL, 6 times higher than the 90% receptor occupancy concentration in vitro. The C max,ss and AUC ss under the 400 mg Q6W regimen were lower than the observed maximum administered dose (10 mg/kg). Results of the univariate logistic regression analyses evaluating relationships between efficacy (CR, ORR and DCR) and PK exposure (simulated C max,ss, C min,ss, AUC ss) demonstrated a relatively flat ER relationship without dose dependency in R/R cHL patients. The relationship between PK exposure (the same as above) and safety response (≥ grade 3 TRAE, ≥ grade 3 irAE or AE leading to suspension) were also analyzed in patients with different types of cancer, and the result showed no apparent correlation between safety and drug exposure.
Conclusions:
PopPK simulations and ER analyses indicate that both weight-based dose and fixed-dose (200 mg Q3W or 400 mg Q6W) are appropriate for penpulimab.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal