Abstract
Introduction:
Multicentric Castleman disease (MCD) is a rare lymphoproliferative disorder characterized by lymph node enlargement in multiple regions of the body. It includes two heterogeneous subtypes: human herpesvirus 8 (HHV-8) associated MCD and idiopathic MCD (iMCD). This study is focused on the efficacy and safety of treatment regimens for MCD in clinical trials.
Methods:
A systematic literature search was conducted using PubMed, Embase, Cochrane, Web of Science, Clinicaltrials.gov, ASH, and ASCO meeting websites from inception to May 9, 2021. The initial search revealed 1323 articles. After excluding reviews, duplicates, and non-relevant articles, we included data from 13 articles.
Results:
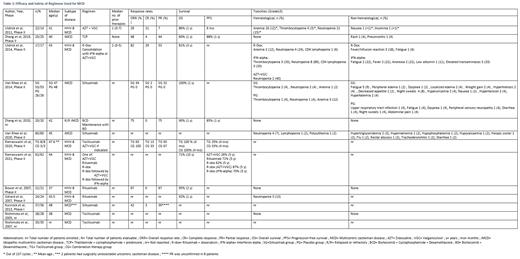
Data on 311 patients was included.
Siltuximab based regimens:
In the two clinical trials where siltuximab was used for iMCD, Cheson criteria (modified to include cutaneous lesions) was used to assess response. In a phase 2 trial by Van Rhee et al. (2014, n=79), the overall response rate (ORR) was 34% vs 0% and grade≥3 adverse events (AEs) were seen in 47% vs 54% of the siltuximab (n=53) and placebo (n=26) groups respectively. In a phase 1 trial by Kurzrock et al. (2013, n=37), 86% of patients improved in at least one clinical benefit response (CBR) component, 33% had confirmed radiological ORR, and 11% had grade≥3 AEs. Van Rhee et al. (2020, n=60) conducted an extension analysis of patients from these two trials. After a median follow-up of 6 years (IQR 5∙11-7∙76), serious AEs were reported in 42% of patients and stable disease (SD) or better of up to 6 years was recorded in 70% of patients.
Tocilizumab based regimens:
In a phase 2 trial by Ramaswami et al. (2020, n=8), ORR was 63% vs 100%, progression-free survival (PFS) at 4 months was 25% vs 33%, and grade≥3 AEs were reported in 25% vs 67% of HHV-8 associated MCD patients receiving tocilizumab alone vs tocilizumab alongside zidovudine (AZT) and valganciclovir (VGC), respectively. Nishimoto et al. (2005, n=28) reported a 30% reduction (p<0.001) in the mean short axis of swollen lymph nodes, after 1 year of therapy. The 5 year extension study by Nishimoto et al. (2007, n=35) reported 2.3 AEs / patient year.
Rituximab based regimens:
In a phase 2 trial by Bower et al. (2007, n=21), 67% of HIV-associated MCD patients had a partial response (PR) and 29% had SD on rituximab. The 2-year overall survival (OS) and relapse-free survival were 95% (95% CI, 86% to 100%) and 79% (95% CI, 52% to 100%) respectively. No grade≥3 toxicity was observed. In a phase 2 trial by Gerard et al. (2007, n=24) on rituximab in HIV-associated MCD, the sustained remission rate was 92% (95% CI, 80% to 103%) at day 60. Estimated 1-year OS was 92% (95%CI, 71% to 98%). In a phase 2 trial by Uldrick et al. (2014, n=17), rituximab and doxorubicin (R-dox) were administered to HHV-8 MCD patients. ORR was 82% and OS was 81% (95%CI, 57% to 93%) at 3 years. Ramaswami et al. (2021) reported survival outcomes from the same trial. The 5-year PFS with rituximab (n=8), R-dox followed by no maintenance (n=16), R-dox followed by AZT+VGC (n=10) and R-dox followed by interferon-alpha (n=10) were 71% (95%CI, 26% to 92%), 62% (95%CI, 23% to 85%), 87% (95%CI, 39% to 98%) and 70% (95%CI, 33% to 89%) respectively.
Immunomodulatory drug-based regimens:
In a phase 2 study by Zhang et al. (2019, n=25), ORR was 48% in iMCD patients on thalidomide, cyclophosphamide, and prednisone. Estimated 1-year OS and PFS were 88% and 60% respectively. Grade≥3 AEs were rash and pneumonitis in 1 (4%) patient each.
Proteasome Inhibitor based regimens:
Zhang et al. (2020, n=20) reported the efficacy of bortezomib, cyclophosphamide, and dexamethasone in relapsed and refractory (R/R) iMCD patients. Seventy-five percent had PR and 10% had SD. Estimated 1-year PFS and OS were 85% and 90% respectively. No grade≥3 AEs occurred.
Antiviral Regimens:
In a phase 2 trial by Uldrick et al. (2011, n=14), ORR was 28% in HHV-8 MCD patients on AZT and VGC. Clinical complete response (CR) occurred in 50% of patients. Ramaswami et al. (2021) reported the efficacy of AZT+VGC in 17 patients from the same trial. The 5-year PFS was 26% (95%CI, 8% to 49%).
Conclusion:
Siltuximab- and thalidomide-based regimens have acceptable efficacy and safety profiles for the treatment of iMCD. Bortezomib appears to be effective for R/R iMCD. Tocilizumab and rituximab showed promising outcomes for the treatment of HHV-8 associated MCD. However, more prospective clinical trials need to be conducted.
Anwer: Allogene Therapeutics: Research Funding; BMS / Celgene: Honoraria, Research Funding; GlaxoSmithKline: Research Funding; Janssen pharmaceutical: Honoraria, Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal