Abstract
Background:
Ropeginterferon alpha-2b (Ropeg) is a novel pegylated interferon-alpha approved for the treatment of polycythemia vera (PV) in Europe and Taiwan. Prior to its approval in Taiwan, the major options for patients with myeloproliferative neoplasms (MPNs) included hydroxyurea (HU) and/or anagrelide. Patients who are HU/anagrelide resistant/intolerant have limited options, as ruxolitinib is not subsidized by the national health insurance in Taiwan for PV. In 2017, the manufacturer provided a compassionate use program (CUP) for patients who were HU and/or anagrelide resistant/intolerant. Herein, we assessed the efficacy and safety of Ropeg in 20 MPN patients.
Methods:
To be eligible, patients must be resistant or intolerant to currently available therapies for MPN in Taiwan, mainly HU and anagrelide. Patients with autoimmune disorders, psychiatric illness, and acute/chronic infections were excluded. An accelerated dosing regimen was used, starting from 250 µg, and increased by 100 µg every two weeks until it reached the target dose of 500 µg, if no self-reported discomforts or abnormalities in biochemical or hematological profiles were observed. Efficacy assessments included hematologic parameters, phlebotomy need, and JAK2V617F allele burden. Hematologic remission was defined as platelets ≤400 x 10^9/L and white blood cells <9.5 x 10^9/L for essential thrombocythemia (ET), and platelets ≤400 x 10^9/L, white blood cells <10 x 10^9/L, and hematocrit <45% with no phlebotomy in the past 3 months for PV. Molecular response was defined as a reduction in JAK2V617F allele burden of at least 50% from baseline if baseline value was less than 50%, and a reduction of at least 25% from baseline if the baseline level was at least 50%. Each case was independently reviewed and approved for the use of Ropeg by both Institutional Review Board and the Ministry of Health and Welfare in Taiwan.
Results:
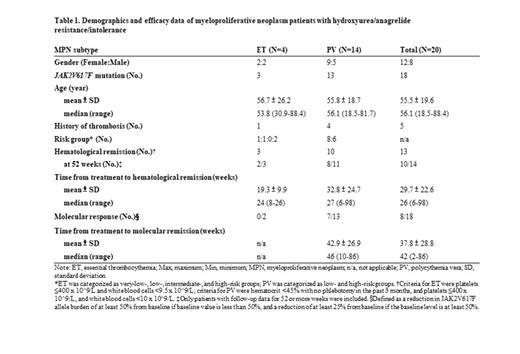
A total of 20 patients received treatment, which included 14 PV, 4 ET, 1 post-ET myelofibrosis (MF), and 1 pre-fibrotic primary MF (Table 1). There were 12 female and 8 male patients with a median age of 56.1 years old. Of these 20 patients, 18 had JAK2V617F mutation and 5 had a history of thrombosis. Of the 18 ET and PV patients, 13 achieved hematologic remission. The ET patients seemed to achieve hematologic remission faster than PV patients (19.3 vs. 33.2 weeks). Of the 18 patients with JAK2V617F mutation, 7 PV patients and 1 post-ET MF patient achieved molecular response, which took a median of 46 weeks after Ropeg treatment. Reduction in JAK2V617F allele burden was observed in 12 patients. One MF patient discontinued treatment due to disease progression. Another PV patient discontinued treatment due to acute myeloid leukemia transformation, although after treatment, the patient returned to PV state and continued Ropeg treatment. Overall, the drug was well tolerated, as most of the treatment-related adverse events (AEs) were mild to moderate. The AE profile was consistent with those from the phase 3 PROUD/CONTI-PV study. There were no unbearable side effects that led to treatment discontinuation.
Conclusion:
Our study provided evidence in the efficacy and safety of Ropeg for the treatment of HU-/anagrelide-resistant/intolerant MPNs. Hematologic remission was observed in ET and PV patients, whereas molecular response was observed in only PV patients, possibly due to the small sample size of ET patients. Our experience with Ropeg suggests it to be a promising option for the treatment of MPNs with drug-resistance/intolerance.
Chen: PharmaEssentia: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Amgen, Celgene, Novartis, and Panco Healthcare: Honoraria. Shih: PharmaEssentia Co: Consultancy, Membership on an entity's Board of Directors or advisory committees; Celgene Ltd: Research Funding; Ltd: Research Funding; Novartis: Research Funding.
Ropeginterferon alfa-2b is a novel interferon alpha indicated for the treatment of polycythemia vera in Europe and in Taiwan. This abstract describes the use of this agent for the treatment of myeloproliferative neoplasm patients with hydroxyurea/anagrelide resistance/intolerance.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal