Abstract
Introduction
Systemic immunoglobulin light chain (AL) amyloidosis is a rare disease characterized by amyloid fibril deposits, most commonly in the heart and kidneys. Management of the disease relies primarily on off-label use of multiple myeloma therapies. No treatments are approved for AL amyloidosis in the relapsed/refractory setting. In the phase 3, open-label TOURMALINE-AL1 trial (NCT01659658), patients with relapsed/refractory AL amyloidosis were randomized 1:1 to receive either oral ixazomib plus dexamethasone (Id) or physician's choice (CH) in 28-day cycles, and followed until progression or unacceptable toxicity. An interim analysis demonstrated that Id was well tolerated and, although the primary endpoint of hematologic response rate was not met, time-to-event efficacy analyses consistently favored Id. We report an analysis of patient-reported outcome (PRO) data on health-related quality of life (HRQOL) and AL amyloidosis symptoms from TOURMALINE-AL1; such data for AL amyloidosis in general have been limited.
Methods
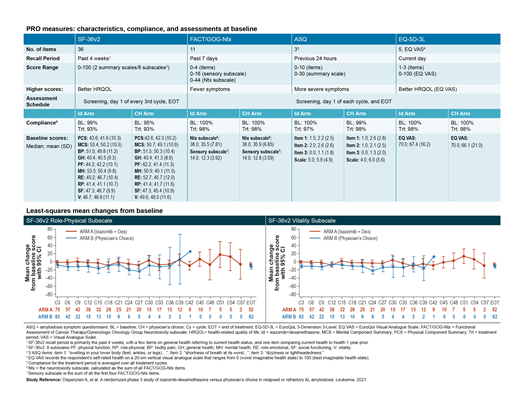
Four PRO instruments were used to collect data to support PRO-related secondary endpoints during the treatment period: the Functional Assessment of Cancer Therapy/Gynecologic Oncology Group Neurotoxicity subscale (FACT/GOG-Ntx), an amyloidosis symptom questionnaire (ASQ), the EuroQoL 5-Dimension 3-Level (EQ-5D-3L), and the Visual Analogue Scale (EQ VAS) were collected at screening, day 1 of each cycle, and end of treatment (EOT), and the 36-item Short Form General Health Survey version 2 (SF-36v2) was collected at screening, day 1 of every third cycle, and EOT. Analysis of PRO data included descriptive summaries of means and change from baseline and a linear mixed model (LMM) for repeated measures for patients in the intent-to-treat (ITT) population with a baseline and at least one postbaseline PRO-specific assessment. No adjustment was made for multiple comparisons. Data cutoff date was 20 February 2019.
Results
The ITT population included 168 patients (Id: n=85; CH: n=83), who completed up to 58 cycles (Id) and up to 43 cycles (CH); median treatment duration was 11.7 vs 5.0 months, respectively. PRO compliance in the treatment period was high for all instruments (Figure).
Baseline HRQOL as measured by the SF-36v2 and EQ VAS was similar between treatment arms. During the treatment period, SF-36v2 Mental and Physical Component Summary (MCS and PCS) scores remained stable relative to baseline and were similar between arms; the 8 subscale scores were also generally maintained over time, and mostly similar between arms. Least-squares (LS) mean changes from baseline were significantly higher (better HRQoL) for Id compared with CH at several cycles in the SF-36v2 Role Physical and Vitality subscales (p<0.05) (Figure); no subscales demonstrated significant differences favoring CH.
Symptom burden as measured by the FACT/GOG-Ntx and ASQ was low at baseline, leaving little room for improvement. FACT/GOG-Ntx scores were generally maintained over time. In the treatment period, there were small but significant differences in LS mean changes from baseline favoring Id over CH at multiple cycles for 7 of the 11 individual items and the neurotoxicity and sensory summary scores; there were small but significant differences favoring CH over Id for 1 individual item (trouble hearing) at a few later cycles.
The ASQ total score trended downward slightly (lower burden) during treatment after the first few cycles in both arms. There were significant differences per the LMM favoring Id over CH at cycles 7 and 21 (p<0.05).
In both arms, EQ VAS scores trended upward (better HRQoL) during treatment and then declined slightly at EOT. (LMM analyses were not conducted for EQ-5D.)
At EOT, a slight deterioration from baseline was observed in actual scores for SF-36v2 domains and summary scales, FACT/GOG-Ntx neurotoxicity and sensory subscales, and ASQ items and total score for both Id and CH.
Conclusions
Relapsed/refractory AL amyloidosis patients treated with Id experienced HRQOL and symptoms that were similar to or trended better than patients treated with CH. These data suggest that treatment with Id, although with a substantially longer treatment duration, did not have a negative impact on HRQOL in patients with relapsed/refractory AL amyloidosis, a population with currently no approved treatment options.
Sanchorawala: Regeneron: Membership on an entity's Board of Directors or advisory committees; Proclara: Membership on an entity's Board of Directors or advisory committees; Oncopeptide: Research Funding; Karyopharm: Research Funding; Sorrento: Research Funding; Pfizer: Honoraria; Caelum: Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Membership on an entity's Board of Directors or advisory committees; Prothena: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Research Funding; Celgene: Research Funding. Wechalekar: Caelum Biosciences: Other: Clinical Trial Funding; Alexion, AstraZeneca Rare Disease: Consultancy; Janssen: Consultancy; Celgene: Honoraria; Amgen: Research Funding; Takeda: Honoraria. Kim: Janssen, BMS: Research Funding. Schönland: Janssen: Honoraria, Other: Travel grants, Research Funding; Takeda: Honoraria, Other: Travel grants; Sanofi: Research Funding; Prothena: Honoraria, Other: Travel grants; Pfizer: Honoraria. Landau: Takeda, Janssen, Caelum Biosciences, Celgene, Pfizer, Genzyme: Membership on an entity's Board of Directors or advisory committees; Takeda: Research Funding; Genzyme: Honoraria. Fiona: Pfizer: Research Funding. Suzuki: Janssen: Consultancy, Honoraria; Abie: Honoraria; Sanofi: Honoraria; Novartis: Honoraria; ONO: Honoraria; Takeda: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Honoraria, Research Funding. Dispenzieri: Sorrento Therapeutics: Consultancy; Oncopeptides: Consultancy; Pfizer: Research Funding; Alnylam: Research Funding; Janssen: Consultancy, Research Funding; Takeda: Research Funding. Comenzo: Karyopharm: Research Funding; Takeda: Research Funding; Unum: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi-Aventis: Membership on an entity's Board of Directors or advisory committees; Janssen: Patents & Royalties: WO2016187546A1, Research Funding; Prothena Biosciences: Consultancy, Research Funding; Caelum: Consultancy, Research Funding. Cherepanov: Takeda: Current Employment, Current equity holder in publicly-traded company. Hayden: Takeda: Current Employment, Current equity holder in publicly-traded company. Kumar: Takeda: Current Employment, Current holder of stock options in a privately-held company. Labotka: Takeda: Current Employment. Faller: Takeda Pharmaceuticals Co.: Current Employment; Viracta Therapeutics, Inc: Current holder of stock options in a privately-held company, Ended employment in the past 24 months, Membership on an entity's Board of Directors or advisory committees; Briacell, Inc: Current holder of stock options in a privately-held company. Kastritis: Amgen, Genesis Pharma, Janssen, Takeda: Consultancy; Amgen, Janssen, Pfizer: Research Funding; Amgen, Genesis Pharma, Janssen, Takeda, Pfizer: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal