Abstract
Intro: Hematopoietic stem-cell transplant (HSCT) recipients are considered to be at high risk for poor outcomes following COVID-19 infection given their co-morbidities and immunosuppression. Sharma et al published the CIBMTR observational report data that showed that recipients of allogeneic HSCT who contract COVID-19 have poor overall survival with a 30-day mortality of 32%. That being said, there have been relatively few studies that look into the effect of COVID-19 on HSCT recipients in the setting of in vivo T cell depletion protocols. With the increased use of post-transplant cyclophosphamide (PTCy) based GVHD prophylaxis regimens for our match related and match unrelated HSCT recipients since 2018 at our institution, we are interested to see if our COVID-19 outcomes differ from those published in the CIBMTR report.
Methods: This is a single institution retrospective analysis evaluating outcomes of HSCT recipients who were diagnosed with COVID-19 between March 2020 and April 2021. Patients 18 years or older who underwent HSCT and subsequently contracted COVID-19 were included in the data collection. Demographic data including age, type of hematologic malignancy, conditioning regimen, GVHD prophylaxis, date of COVID-19 infection, with pre- and post-COVID-19 infection labs were obtained. Our primary endpoint in this retrospective analysis was non-relapse mortality within 30 days of COVID-19 diagnosis.
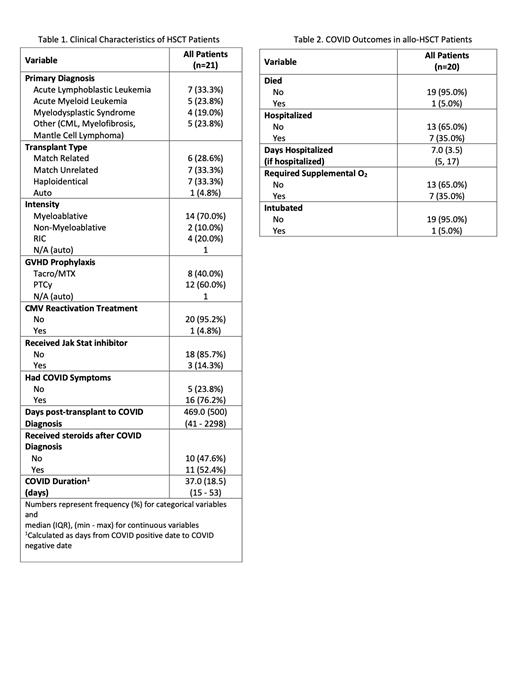
Results: There were 21 patients at our institution who had undergone HSCT and subsequently contracted COVID-19. The most common primary disease types were acute lymphoblastic leukemia (33.3%), acute myeloid leukemia (23.8%), and myelodysplastic syndrome (19.0%). The median age of our patient population was 53 years (range, 24-66). 6 of the patients received match related allografts. 7 received cells from match unrelated donors. 7 received cells from haploidentical donors. 1 patient had received an autologous stem cell transplant. Of the remaining 20 allo-HSCT recipients, 14 of them (70.0%) received myeloablative conditioning regimens, whereas 6 (30.0%) received reduced intensity or non-myeloablative regimens. Our GVHD prophylaxis regimens were PTCy/Tacro/MMF (12 pts, 60.0%) and Tacro/MTX (8 pts, 40.0%).
Patient demographics and outcomes are found on Tables 1 and 2. Our patients were diagnosed with COVID-19 a median 469 days post-transplant, with 8 patients (38.1%) diagnosed with COVID-19 within 1 year of transplant. 11 of the patients (52.4%) received steroids following their diagnosis with COVID-19. Of the 20 allo-HSCT recipients with confirmed COVID-19 infection, 1 passed away 20 days after the diagnosis was made. This gives us a 5.0% case fatality rate attributable to COVID-19 in our population in our allo-HSCT population. 16 of the 20 patients were symptomatic at the time of diagnosis (80.0%). 7 of the 20 patients (35.0%) were hospitalized for a median of 7 days (range, 5-17 days), with 2 requiring ICU level of care. The one patient who passed away tested positive for COVID-19 177 days post-transplant and was hospitalized approximately 7 days after diagnosis, where he was intubated on hospital day 4 and ultimately passed away on hospital day 13. The patient had received Tacro/MTX for GVHD prophylaxis.
Discussion: Although this is a small sample size, our data suggests that our allo-HSCT recipients who contracted COVID-19 have had generally good short-term outcomes. Our study is limited by the small number of patients who got infected with COVID-19, particularly those within 1-year post-transplant. Furthermore, we acknowledge it is difficult to claim that the PTCy based GVHD prophylaxis regimens for our HSCT recipients were solely responsible for their improved outcomes since 40% of allo-HSCT recipients did not get PTCy for GVHD prophylaxis. However, we believe it would be valuable to evaluate in a prospective analysis. We are currently evaluating if a COVID-19 diagnosis has any effect on long term transplant related complications and outcomes in this population.
Chaudhary: Angeles Therapeutics: Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Other: Founder, Patents & Royalties: Cell therapy ; Celldex: Current equity holder in publicly-traded company; Moderna: Current equity holder in publicly-traded company; Pancella: Consultancy; Oncotartis: Consultancy; Athelas: Consultancy, Current holder of stock options in a privately-held company; TCR2: Current equity holder in publicly-traded company; Allogene: Current equity holder in publicly-traded company. Yaghmour: Novartis: Consultancy, Speakers Bureau; BMS: Speakers Bureau; Alexion: Speakers Bureau; Astellas: Speakers Bureau; Takeda: Consultancy, Speakers Bureau; Jazz: Speakers Bureau; Agios: Consultancy, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal