Abstract
Background
Beti-cel ex vivo gene therapy integrates a modified HBB gene into hematopoietic stem cells of patients with TDT, aiming to enable lifelong, stable production of functional adult hemoglobin (Hb). The efficacy and safety of the treatment have been demonstrated in a total of 63 patients treated across 4 clinical trials (HGB-204,-HGB-205, HGB-207, and HGB-212). Here, we present the first patient who received beti-cel outside of the clinical trial setting, a 14-year-old male with a β 0/β + (IVS-1-6) genotype.
Methods
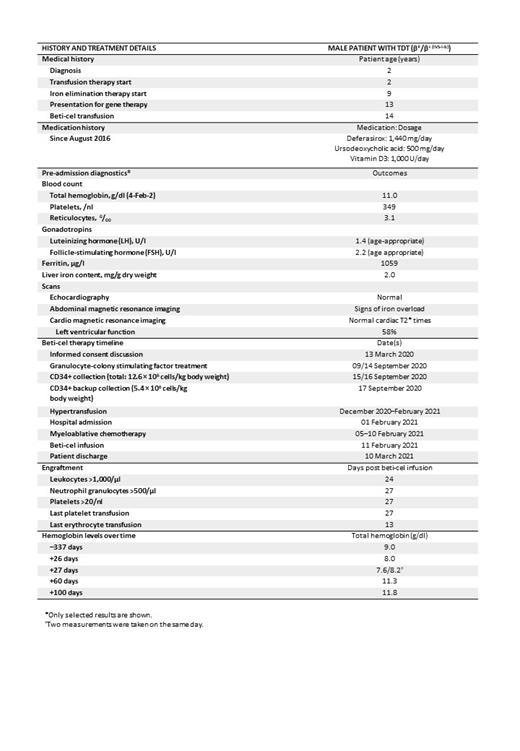
Following hematopoietic stem cell collection via granulocyte-colony stimulating factor plus plerixafor mobilization and apheresis, CD34+ cells were transduced with the BB305 lentiviral vector encoding HbA T87Q. The patient received hypertransfusion before mobilization and conditioning, maintaining a pre-transfusion Hb level of >11 g/dL. Six days prior to beti-cel infusion, single-agent busulfan myeloablation was initiated (16 single doses at 0.8 mg/kg body weight; 3.2 mg/kg/24 h) with concomitant clonazepam (see Table for treatment timeline). Ursodeoxycholic acid therapy was continued as hepatic veno-occlusive disease (VOD) prophylaxis through inpatient treatment.
Results
The patient was diagnosed with TDT at the age of 2 years in his home country and has been treated in Germany since the age of 9. Regular transfusion therapy was initiated soon after diagnosis (Table). Aged 9, the patient was started on desferasirox for iron elimination therapy. His annualized red blood cell (RBC) transfusion volume was 174 ml/kg in 2018 and 185 ml/kg in 2019, maintaining his pre-transfusion Hb at or above 9 g/dl. No HLA-related donor was available for allogeneic transplant.
At informed consent, the patient was 13 years old and met the eligibility criteria for beti-cel treatment as outlined in the summary of product characteristics (SmPC). The patient was physically fit, with a 90% Lansky score and regular participation in school sports, but reported physical limitations when running extensively. The patient underwent a thorough assessment before admission (Table), which did not reveal any remarkable abnormalities except TDT-related splenomegaly and signs of slight iron overload (liver iron content, 2.0 mg/g dry weight [normal range, 0.17-1.8]).
On 11/Feb/2021, the patient was infused with 5.1 × 10 6 CD34+ cells/kg. The patient received 4 RBC and 8 platelet transfusions following infusion until Day 13 and 27, respectively (Table). Neutrophil and platelet engraftment occurred on day 27 post beti-cel infusion. The patient was discharged from inpatient treatment the same day, in excellent general condition, with 90% Lansky score, Hb of 8.2 g/dl, a reticulocyte count of 9.3%, a total white cell count of 1.55/nl, a neutrophil count of 0.75/nl, and a platelet count of 24/nl.
At last follow-up (+100 days), the patient felt well and exhibited normal exercise tolerance. He has received neither red blood cell nor platelet transfusions or chelation therapy since discharge. Total Hb was 11.8 g/dl (Table). Granulocytes and lymphocytes had recovered to normal levels. The patient showed continued, albeit slowly improving, thrombocytopenia (platelet count, 31/nl [29/nl at +60 days]), consistent with previous observations after beti-cel therapy.
Myeloablation and beti-cel infusion were tolerated well. Adverse events post infusion were febrile neutropenia, elevated C-reactive protein levels, pruritus, gingivitis, mild mucositis, and vertigo, consistent with the SmPC. At +23 and +26 days, the patient experienced transient subjective hearing loss (quickly resolved). No VOD events occurred.
Conclusions
This is the first real-world patient with TDT treated with beti-cel therapy. The treatment regimen had a tolerability profile consistent with that of mobilization, apheresis, and busulfan myeloablation, matching clinical trial observations. Following treatment, this 14-year-old patient reached a total Hb of 11.8 g/dL at +100 days without requirement of red cell transfusions and continues to exhibit prolonged but slowly improving and asymptomatic thrombocytopenia.
Schmitt: TolerogenixX Ltd: Current Employment; Therakos/Mallinckrodt: Research Funding; Hexal: Other: Travel grant; Jazz Pharmaceuticals: Other: Travel grant. Schmitt: Bluebird Bio: Other: Travel grants; Novartis: Other: Travel grants, Research Funding; TolerogenixX: Current holder of individual stocks in a privately-held company; Apogenix: Research Funding; MSD: Membership on an entity's Board of Directors or advisory committees; Hexal: Other: Travel grants, Research Funding; Kite Gilead: Other: Travel grants. Kulozik: Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sanofi: Consultancy, Honoraria; BioMedX: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; bluebird bio, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal