Abstract

Background: Patients with relapsed or refractory (R/R) DLBCL generally have a poor prognosis, particularly if they are not candidates for autologous stem cell transplantation (ASCT) or experience relapse following approved CAR-T therapies. The UNITY-NHL study systematically explored the efficacy and tolerability of the PI3K-d/CK1-e inhibitor, umbralisib, alone (umbra), and in combination with the glycoengineered anti-CD20 monoclonal antibody ublituximab (U2), followed by a cohort treated with U2 plus bendamustine (U2+benda). Herein we report on the experience in a large cohort of patients with R/R DLBCL.
Methods: This study explored a sequential combination design as described above. Eligible patients had histologically confirmed R/R DLBCL and were ineligible for ASCT, with no limit on number or type of prior treatment. Umbra was given orally at 800 mg once daily in 28-day cycles (C) until disease progression or unacceptable tolerability. Ublituximab was administered intravenously (IV) on Days 1, 8, and 15 of C1, on Day 1 of C2-6, and on Day 1 every 3C through C24. Benda was administered IV (90 mg/m 2) on Days 1/2 of C1-6. Cell of origin, NGS, and c-myc (FISH) were analyzed centrally. The primary endpoint of the study was overall response rate (ORR) as assessed by an independent review committee. Secondary endpoints included duration of response, progression-free survival, time to response, and safety.
Results: 226 patients with DLBCL were enrolled as follows: umbra monotherapy (n=30), U2 (n=66), and U2+benda (n=130). The population demographics included the following features: median age was 72 years (range 32-95); 59% were male; 64% of patients had stage III or IV disease; 58% were refractory to their immediate prior therapy; and the median number of prior therapies was 2 (range 1-8). There were no substantive differences in these characteristics across cohorts.
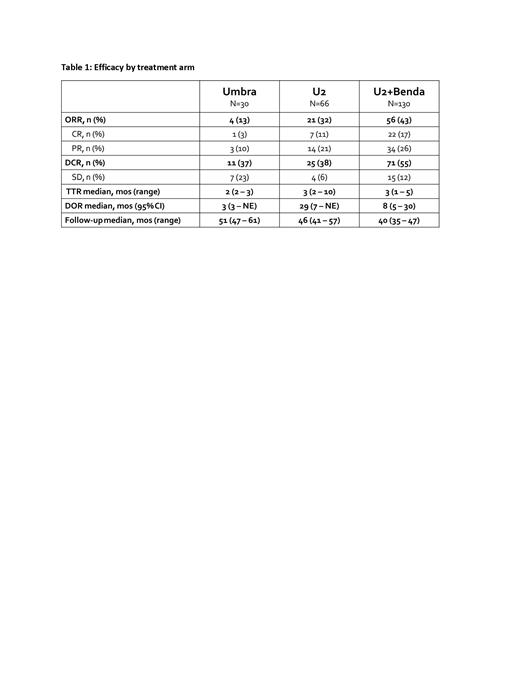
Median follow-up for the umbra, U2, and U2+benda arms was 51 months (range 47-61), 46 months (range 41-57), and 40 months (range 35-47), respectively. Overall and complete response rates for the umbra mono, U2, and U2+benda arms were 13.3% (CR 3.3%), 31.8% (CR 10.6%), and 43.1% (CR 16.9%), respectively. Results pertaining to secondary endpoints are listed in Table 1. Correlation of response to cell of origin and mutation/c-myc status is ongoing and will be available at the time of presentation.
Adverse events (AEs) were similar across the cohorts, with the exception of hematologic AEs which were increased in patients receiving benda. The most common all-grade AEs by treatment arm (umbra, U2, and U2+benda, respectively) were diarrhea (47%; 41%; and 48%), nausea (40%; 45%; and 45%), fatigue (33%; 30%; and 41%) and neutropenia (3.3%; 18%; and 32%). All-grade AEs of special interest included non-infectious colitis (3.3%, 1.5%, and 2.3%) and pneumonitis (3%, 1.5%, and 1.5%) in umbra, U2 and U2+benda treated patients respectively. Grade 3/4 AEs were uncommon, with the only events >10% being limited to neutropenia (11% for U2; 27% for U2+benda), and anemia (17% for U2+benda).
Conclusions: In the DLBCL cohort of UNITY-NHL, the U2+benda triplet regimen was active and well tolerated in patients with R/R DLBCL who were unsuitable for transplant or who had relapsed following ASCT. Umbra monotherapy and U2 were also well tolerated but resulted in lower ORR than in the U2+benda cohort.
Burke: X4 Pharmaceuticals: Consultancy; Bristol Myers Squibb: Consultancy; SeaGen: Consultancy, Speakers Bureau; Beigene: Consultancy, Speakers Bureau; Roche/Genentech: Consultancy; Epizyme: Consultancy; Adaptive Biotechnologies: Consultancy; Kura: Consultancy; MorphoSys: Consultancy; AstraZeneca: Consultancy; Kymera: Consultancy; AbbVie: Consultancy; Verastem: Consultancy. Fonseca: Amgen: Honoraria, Speakers Bureau; Celgene/BMS: Honoraria, Speakers Bureau; Dava Oncology: Honoraria; Epizyme: Honoraria; Karyopharm: Honoraria; Sanofi: Honoraria; Abbvie: Honoraria. Jurczak: Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novo Nordisk: Research Funding; Morphosys: Research Funding; Mei Pharma: Research Funding; Merck: Research Funding; Loxo Oncology: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Research Funding; Epizyme: Research Funding; Debbiopharm: Research Funding; Celgene: Research Funding; Celtrion: Research Funding; BeiGene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bayer: Research Funding; Astra Zeneca: Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Research Funding; Sandoz: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Research Funding; TG Therapeutics: Research Funding. Melear: TG Therapeutics: Speakers Bureau; Astrazeneca: Speakers Bureau; Janssen: Speakers Bureau. Islas-Ohlmayer: Seagen Inc.: Research Funding. Reeves: Apollomics, Inc.: Research Funding; Tarveda Therapeutics: Research Funding; Ascentage Pharmaceuticals: Research Funding; Clovis Oncology: Research Funding; Arvinas: Research Funding; Pfizer: Research Funding; Ellipses: Research Funding; ImmunoGen: Research Funding; Karyopharm Therapeutics: Honoraria, Research Funding; Moderna: Research Funding; Thrive: Research Funding; Genentech: Research Funding; Incyte Corporation: Research Funding; Astellas Pharma: Research Funding; IDEAYA Biosciences: Research Funding; Pharmacyclics: Research Funding; Loxo Oncology: Research Funding; AbbVie Inc.: Research Funding; Celgene: Research Funding; GSK: Research Funding; Jiangsu Hengrui Medicine Co.: Research Funding; Arcus Biosciences: Research Funding; Calithera: Research Funding; Amgen: Research Funding; Mirati Therapeutics, Inc.: Research Funding; Array BioPharma Inc.: Research Funding; Taiho Pharmaceutical: Research Funding; Boehringer Ingelheim: Research Funding; GI Therapeutics Inc.: Research Funding; Hutchison: Research Funding; MacroGenics: Research Funding; Ipsen: Research Funding; MedImmune, LLC.: Research Funding; BeiGene: Research Funding; TG Therapeutics: Research Funding; Acerta Pharma: Research Funding; Verastem: Research Funding; Janssen Pharmaceuticals: Research Funding, Speakers Bureau; Eisai Co.: Research Funding, Speakers Bureau; Roche Pharma: Research Funding; Novartis Pharmaceuticals: Research Funding; Daiichi Sankyo: Research Funding; Arvinas: Research Funding; CytomX: Research Funding; Sermonix Pharmaceutical: Research Funding; Seattle Genetics: Research Funding; AstraZeneca: Research Funding; Evelo Biosciences: Research Funding. Wróbel: Takeda: Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau; BMS: Honoraria; Roche: Honoraria, Research Funding, Speakers Bureau; BeiGene: Honoraria; Janssen: Honoraria, Speakers Bureau. Pagel: Incyte/MorphoSys: Consultancy; MEI Pharma: Consultancy; Pharmacyclics/AbbVie: Consultancy; AstraZeneca: Consultancy; Gilead: Consultancy; Actinium Pharmaceuticals: Consultancy; Kite, a Gilead Company: Consultancy; Epizyme: Consultancy; BeiGene: Consultancy. Goldschmidt: Ontada: Current Employment; Blue Ridge Cancer Care: Current Employment; Amgen: Honoraria, Speakers Bureau; BMS: Honoraria, Speakers Bureau; TG Therapeutics: Honoraria; G1 Therapeutics: Honoraria, Speakers Bureau. Miskin: TG Therapeutics, Inc.: Current Employment, Current equity holder in publicly-traded company. Sportelli: TG Therapeutics, Inc.: Current Employment, Current equity holder in publicly-traded company. O'Connor: TG Therapeutics, Inc.: Current Employment, Current equity holder in publicly-traded company; Nomocan: Consultancy; Dren: Consultancy, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company; Myeloid Therapeutics: Consultancy, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company; Kymera: Consultancy, Current equity holder in publicly-traded company; Mundipharma: Consultancy. Ghosh: Seattle Genetics: Consultancy, Honoraria, Speakers Bureau; Genmab: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau; AstraZeneca: Consultancy, Honoraria, Speakers Bureau; Incyte: Consultancy, Honoraria; TG Therapeutics: Consultancy, Honoraria, Research Funding; ADC Therapeutics: Consultancy, Honoraria; Genentech: Research Funding; Karyopharma: Consultancy, Honoraria; Pharmacyclics LLC, an AbbVie Company: Consultancy, Honoraria, Research Funding, Speakers Bureau; Adaptive Biotech: Consultancy, Honoraria; Epizyme: Honoraria, Speakers Bureau; Gilead: Consultancy, Honoraria, Research Funding, Speakers Bureau; AbbVie: Honoraria, Speakers Bureau.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal