Abstract
Background: Sickle cell nephropathy (SCN) is one of the most common complications of SCD, leading in most cases to chronic kidney disease (CKD) and end-stage renal disease (ESRD). Despite the high prevalence of CKD in sickle cell disease (SCD) patients, there remains a poor understanding of the pathophysiological mechanism of SCN and a lack of biomarkers for early detection of SCD-associated CKD. Soluble urokinase-type plasminogen activator receptor (suPAR) is an emerging biomarker of CKD. suPAR is a member of the fibrinolytic system, which is dysregulated in SCD patients.
Objective: To evaluate suPAR as a biomarker of SCD-associated nephropathy and identify plasma proteases responsible for its increase in SCD.
Methods: The study was approved by Howard University review board (IRB) and all subjects provided written inform consent prior to the sample collection. Whole blood and urine samples were collected from 77 SCD patients and 10 healthy individuals, and plasma was isolated. Levels of creatinine and cystatin C in plasma and albumin and creatinine in urine were measured by ELISA. eGFR was calculated using CKD-EPI creatinine-cystatin equation, and CKD stages were assigned. Plasma suPAR was measured by ELISA and was correlated with CKD stages. The activities of candidates uPAR proteases: Neutrophile elastase (NE), urokinase-type plasminogen activator (uPA) and plasmin in plasma samples from SCD patients were measured and compared to healthy participants.
Results:
The average age of SCD patients was 42.5 years (range 18-67 years). Most patients had HbSS genotype (67.5%),19.5% of patients were HbSC (hemoglobin C sickle cell compound heterozygous), and 13% had HbS β-thalassemia. More than half (53.2 %) were females.
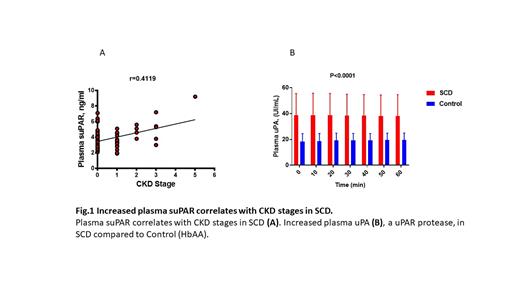
We observed an increased level of plasma suPAR (>3ng/ml) in more than 60% of SCA patients without renal disease, representing a risk factor for CKD progression. Plasma suPAR levels further increased in the patients with CKD and positively correlated with stages of CKD (r=0.419, R2=0.1696). Analysis of plasma proteases that cleaved uPAR producing soluble peptides (suPAR) demonstrated increased urokinase-type plasminogen activator (uPA) activity without significant changes in neutrophile elastase.
Conclusion:
This study validated plasma suPAR as a potential marker of CKD in SCD patients and identified plasma uPA as a uPAR protease that may increase circulating suPAR in SCD. Future longitudinal analysis of suPAR levels in patients with SCA is needed.
Acknowledgments:
We thank Drs. Namita Kumari and Xiaomei Niu for their help in samples identification. This work was supported by NIH Research Grants 1R01HL125005-06A1, 5U54MD007597, 1P30AI117970-06,1UM1AI26617, and 1SC1HL150685. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal