Abstract
Background
Worldwide, Sickle Cell Disease (SCD) is the most common single gene disorder affecting >250,000 newborns annually. In Germany, SCD qualifies as a rare disease and almost exclusively affects immigrants from endemic countries and their descendants. The recent surge of immigration from high-prevalence countries increased the numbers of patients with SCD in Germany and raised awareness for the need of specialized care.
In 2012, the German Society for Pediatric Oncology and Hematology (GPOH) mandated a consortium of five university hospitals to develop a disease management program for patients with SCD. This consortium issued treatment guidelines for SCD that strongly favor the use of hydroxyurea, initiated patient and physician education events, prepared for a universal newborn screening program that will start 09/2021, moderated a consensus on the indication of allogeneic stem cell transplantation for patients with SCD, and established a national patient registry.
Methods
In order to quantify the effect of these activities, we made use of claims data that were collected by the research institute (WIdO) of the major German insurance company, the Allgemeine Ortskrankenkasse (AOK), and of publicly accessible data collected by the Federal Statistical Office (Statistisches Bundesamt, Destatis). ICD10 codes were used to identify patients with SCD and their comorbidities. Pharmacologic treatments were quantified using the German Anatomical Therapeutic Chemical (ATC)-Classification with defined daily doses.
Results
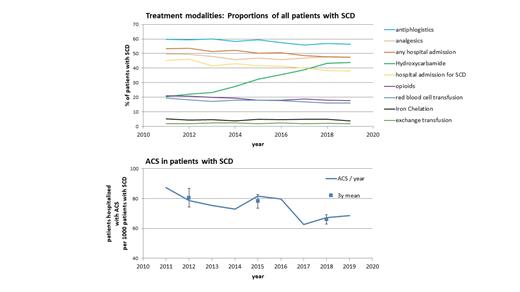
We estimate that the number of patients with SCD in Germany increased from approximately 2,200 in 2011 to approximately 3,200 in 2019. Analyses of administered treatments illustrate that important components of recently issued national treatment guidelines have been largely implemented. Specifically, the use of hydroxyurea has more than doubled, resulting in a proportion of approximately 45% of all patients with SCD being treated with hydroxyurea in 2019 (Figure 1A). In strong negative correlation with the use of hydroxyurea, the frequency of acute chest syndromes decreased (Figure 1B). While before the widespread use of hydroxyurea (2011-2013) 8.1% of patients with SCD were admitted at least once per year for ACS, this proportion dropped to 6.6% in the period 2017 to 2019. Similarly, the proportion of patients who required analgesics, red blood cell transfusions and hospitals admissions declined from 2011 to 2019, indicating a reduced burden of SCD with the increased use of hydroxyurea.
Conclusion
In sum, these data demonstrate an association between the dissemination of nationwide treatment guidelines and changes in clinical practice in particular relating to the use of hydroxyurea. These changes translate into a remarkable improvement of key measures of disease activity in a representative population based analysis.
Lobitz: Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; AddMedica: Honoraria, Membership on an entity's Board of Directors or advisory committees; Vertex: Honoraria, Membership on an entity's Board of Directors or advisory committees. Kulozik: BioMedX: Consultancy, Honoraria; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sanofi: Consultancy, Honoraria; bluebird bio, Inc.: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal