In this issue of Blood, Mahamar et al1 report on predictors of malaria-associated anemia in a prospective study conducted in Malian children living in an area of intense seasonal malaria transmission. Across the African Sahel (the transitional area between the Sahara and the Sudanian savanna), Plasmodium falciparum malaria transmission is intense during the 3- to 4-month rainy season. Anemia is the main manifestation of severe malaria in these areas,2 seen mainly in young children. During the rains, severe malarial anemia frequently necessitates blood transfusion, and it is an important cause of death in children younger than 5 years of age. In 2020, there were an estimated 627 000 deaths from malaria globally, and a large proportion of those were in children with severe malaria anemia in West Africa.3 Now, in this area, seasonal malaria chemoprevention (monthly treatment courses of amodiaquine-sulfadoxine-pyrimethamine) is given annually during the 3- to 4-month rainy season to more than 20 million children between 3 and 59 months of age to prevent malaria and its adverse consequences. During the rainy season, malaria is ubiquitous; in many areas, mosquitoes inoculate inhabitants of this area with P falciparum parasites several times per week. Many of these acquired infections are asymptomatic because disease-controlling immunity is acquired during childhood. But some infections cause illness, and a few are lethal. Even after hospital admission for severe malarial anemia, there is still high mortality following discharge, which may be prevented by malaria chemoprophylaxis.4 Severe malarial anemia is also associated with long-term neurocognitive impairment, especially in young children.5
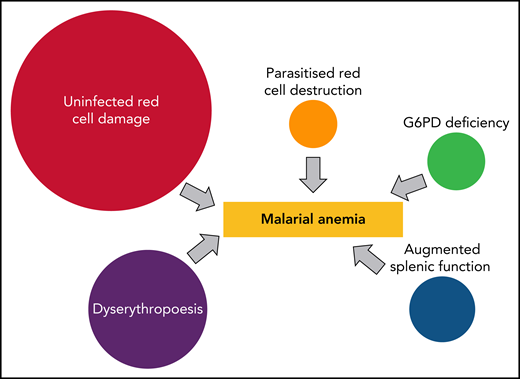
So why is it that some malaria infections cause severe anemia, whereas others cause very little change in hemoglobin concentrations? In repeatedly infected children, there is little time for recovery, and progressive anemia ensues. In some other patients, a single infection causes a precipitous fall in hemoglobin.2,6 There are 3 concomitant pathological processes (see figure).7 First, there is the predominant clearance of unparasitized erythrocytes. Various factors are thought to contribute to this bystander destruction. These include generalized reduced red cell deformability, which may result from oxidative damage, increased splenic clearance function, and, in some cases, increased complement-mediated clearance. Second, there is bone marrow dyserythropoiesis, and third is the obligatory destruction of parasitized erythrocytes. Each of these processes is proportional to disease severity, with the first 2 reflecting host defense responses. But in most cases, this increased red cell destruction reverses rapidly. In high transmission settings, the hemoglobin usually starts to rise as soon as the infection is controlled by antimalarial treatment. In lower transmission settings, there is a delay of several days before dyserythropoiesis resolves sufficiently for reticulocytes to increase, and for the hemoglobin to rise.
Direct contributors to malarial anemia. The proportions vary but, in general, uninfected erythrocyte hemolysis is the major contributor to acute anemia, whereas dyserythropoiesis is a major contributor to subacute anemia in children in high transmission settings.
Direct contributors to malarial anemia. The proportions vary but, in general, uninfected erythrocyte hemolysis is the major contributor to acute anemia, whereas dyserythropoiesis is a major contributor to subacute anemia in children in high transmission settings.
Mahamar et al conducted a pilot quantitative proteomic screen of a very large number of potential blood markers in 9 Malian children with different hematological responses to P falciparum infection to try to understand why these different hematologic responses occur. The lead candidates were then evaluated in a prospective series of more than 70 children from the same cohort. The study excluded children with hemoglobin AS or AC, but did not exclude G6PD deficiency, which may exacerbate malaria-associated hemolysis.8 Higher plasma levels of circulating 20S proteasome and lower levels of insulin-like growth factor-1 were confirmed in children with reduced hemoglobin. The investigators speculated that circulating 20S proteasome may contribute to hemolysis by digesting erythrocyte membrane proteins modified by oxidative stress, whereas decreased insulin-like growth factor-1, which is important for erythroid maturation, might contribute to inadequate erythropoiesis. Of course, association does not necessarily mean causation, so further studies are warranted to elucidate these very interesting findings. These should focus on quantitating the preceding malaria parasite biomass, which is reflected poorly by the parasite density at the time of anemia, and characterizing the sequence of events that preceded the reduction in hemoglobin.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal