In this issue of Blood, Zaiken et al describe the impact on chronic graft-versus-host disease (cGVHD) affecting the formation of germinal centers (GCs) in secondary lymphoid organs (SLOs).1 Using 2 different small molecules targeting polycomb repressive complex 2 (PRC2) or bromodomain and extraterminal (BET)-bromodomain inhibitor, they demonstrate both can improve cGVHD in a murine model of bronchiolitis obliterans (BO) and one also improved scleroderma in another model.
GVHD remains the major complication following allogeneic hematopoietic cell transplantation (HCT). We have come a long way in understanding the pathobiology of this process with novel approaches and drugs that are effective in prevention and therapy. Even with these gains, there is still so much more that is needed. This need is especially pressing for cGVHD because we are transplanting older patients with more comorbidities. Improvements in early treatment-related mortality have led to more patients surviving and at risk for developing cGVHD.
One major insight into the pathology of cGVHD is the role of B cells. B-cell tolerance and signaling are aberrant in cGVHD.2 B cells contribute to cGVHD pathology but are also required for the formation of the lymphoid follicle. In a healthy individual, GCs are structures in the follicles where mature B cells are activated, proliferate, and mature and where somatic hypermutation occurs in response to an antigen. In addition, the follicle is a critical tolerogenic site in SLOs.3 These SLOs are a tightly regulated site of T- and B-cell tolerance induction via exquisite control of a reticular network comprising nonhematopoietic stromal cells, including fibroblastic reticular cells (FRCs).4,5 FRCs play a known pathological role in cGVHD as they fail in their immune tolerogenic role, and they promote aberrant B-cell activation by providing notch ligand and producing B-cell activating factor.6,7 In addition, there are data to suggest that extrafollicular CD4 T- and B-cell interactions are sufficient for inducing cGVHD.8
The authors had previously developed a murine model of multiorgan cGVHD with BO, which is dependent on the formation of GCs. These observations suggest that modulation or inhibition of the GC reaction may be a new therapeutic target. Thus, the investigators set out to test 2 pathways known to be required for GC formation. The first target was enhancer of zeste homolog 2 (EZH2), a histone-lysine N-methyltransferase enzyme that participates in histone methylation and, ultimately, transcriptional repression. EZH2 is the functional enzymatic component responsible for the methylation activity of the PRC2, which is responsible for health through the epigenetic maintenance of genes responsible for regulating development and differentiation. Specifically, PRC2 is a critical regulator in GC formation. A small molecule called JQ5 was used to target EZH2. The second target was directed to BET-bromodomain enzymes, specifically BRD4. These “readers” of the genome recognize acetylated lysine residues and are responsible in transducing the signal and translating it into various normal or abnormal phenotypes. The investigators used another molecule, JQ1, to target this area. In vitro, JQ1 hinders T-cell interleukin-21 expression required for T-follicular helper cell (TFH) function, and in vivo, JQ1 impaired GC B-cell formation via BCL6 repression.
These new data demonstrated that both JQ1 and JQ5 improved pulmonary function, including improvement of lung histology, and, as predicted, these drugs impaired the GC reaction, which was required for the cGVHD, pathological, and clinical changes (see figure). They also demonstrated that JQ5 was on target by demonstrating reduced EZH2-mediated methylation in donor T cells. Using a second model of sclerodermatous cGVHD, JQ5 also reduced the severity of skin changes, but surprisingly JQ1 did not. To determine the mechanistic targets of these 2 drugs, the investigators performed RNA-seq of GC B cells from spleens of cGVHD mice with BO (through all the difficulties during COVID-19). Each drug led to different changes. Moving to gene set enrichment analysis comparing non-cGVHD bone marrow (BM)-only transplant controls vs cGVHD animals, the cGVHD animals demonstrated multiple inflammatory signaling cascades, whereas the BM-only controls had proproliferative gene sets. These proproliferative gene sets were also enriched in the JQ5-treated animals but not in the JQ1-treated animals. Interestingly, neither compound restored a BM-only transcriptomic state; rather, each compound induced a unique set of changes that were independently targeting the function of the GC B cells in cGVHD. The clinical results suggest that reducing splenic frequency of GC cell populations is an attractive approach for cGVHD treatment. The difference in inflammation in different tissues (low in lungs and high in skin) could explain in part the differences in response between the lungs and skin with JQ1 (which was toxic to the animal). These data suggest that drugs such as JQ5 could lead to specific targeting of lesional tissues.
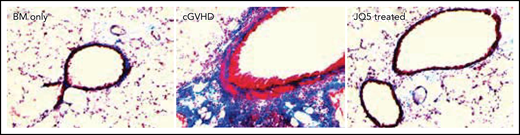
Significant histologic improvement in the lungs of JQ5 treated animals. See the complete Figure 1 in the article by Zaiken et al that begins on page 2983.
Significant histologic improvement in the lungs of JQ5 treated animals. See the complete Figure 1 in the article by Zaiken et al that begins on page 2983.
These are still early days in our understanding of the aberrations in cGVHD and, like layers of an onion, many more questions remain. There is a continuum of changes leading to cGVHD occurring early following HCT specifically owing to the cellular and vascular damage that occurs from the preparatory regimen, especially in the SLO microenvironment. The GVHD microenvironment is composed of a mixture of immune cells and stromal cells with the extracellular matrix proteins. How the stromal cell compartment contributes needs to be investigated further to advance our understanding of the TFH cells and B cells that rely on GCs vs extrafollicular sites for development.9 Inhibition of follicle and GC formation could lead to problems as well. When GCs do not form or FRCs are lost, such as occurs with chronic infection, lymphopenia is perpetuated. The damaged SLO structure contributes to loss of functional T cells. The impact of time after transplant needs to be accounted for, as the setup for cGVHD is likely set up quite early posttransplant. Interventions early on may be beneficial, but the same intervention given later may be deleterious. The authors are to be congratulated on providing a detailed immune analysis coupled with transcriptomic analysis of GC B cells from cGVHD/BO mice. Continued investigation in this area is critical for us to understand the disease process, and we are grateful that these investigators have set the stage for further studies.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal