Abstract
Approximately a third of patients with paroxysmal nocturnal hemoglobinuria (PNH) remain transfusion dependent or have symptomatic anemia despite treatment with a C5 inhibitor. Pegcetacoplan inhibits complement proximally at the level of C3 and is highly effective in treating persistent anemia resulting from C3-mediated extravascular hemolysis. We describe the rationale for C3 inhibition in the treatment of PNH and discuss preclinical and clinical studies using pegcetacoplan and other compstatin derivatives. We propose an approach for sequencing complement inhibitors in PNH.
Introduction
Paroxysmal nocturnal hemoglobinuria (PNH) is a clonal hemolytic anemia that arises following a loss-of-function mutation (PIGA) in hematopoietic stem cells.1PIGA resides on the X chromosome and encodes an enzyme essential for the biosynthesis of glycosylphosphatidylinositol (GPI), a glycolipid that attaches dozens of proteins to the cell surface. PNH may arise de novo or from aplastic anemia. Expansion of the PNH clone is favored in the setting of acquired, but not inherited, forms of aplastic anemia. This is because loss of certain GPI-anchored proteins provides the PNH cell with a conditional advantage in the setting of an autoimmune attack on the stem cell pool (immune escape).2-5 Less commonly, a somatic PIGA mutation arising in the setting of clonal hematopoiesis can lead to PNH without antecedent aplastic anemia.6,7 Pegcetacoplan, the focus of this Spotlight, is a newly approved drug, which blocks complement at the level of C3 and is highly effective in treating PNH.8
Complement dysregulation in PNH
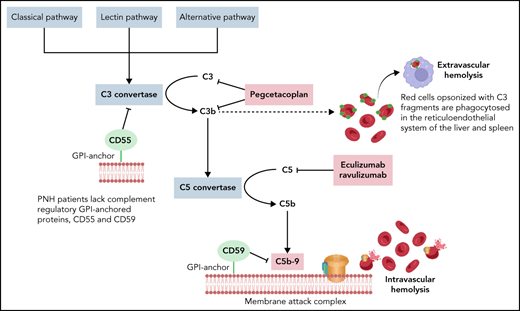
PNH blood cells poorly regulate homologous complement due to the absence of 2 GPI-anchored complement regulatory proteins CD55 and CD59. CD55 regulates the C3 and C5 convertases and CD59 assembly of the membrane attack complex. Mature PNH erythrocytes are most susceptible to complement-mediated intravascular hemolysis because a single membrane attack complex can lyse an enucleated red blood cell.9 PNH patients have chronic hemolysis due to constant low-level complement challenge from the alternative pathway (tickover) but may have severe paroxysms from complement-amplifying conditions associated with infections, surgery, pregnancy, and other inflammatory states. Thrombosis is the leading cause of death from hemolytic PNH without complement inhibition.10,11 Before the 21st century, >40% of PNH patients experienced thrombosis, often involving abdominal veins (portal, hepatic, splenic, mesenteric) or cerebral veins.12 Arterial thrombi may also occur but are less common. Therapeutic anticoagulation, without complement inhibition, did not reliably prevent thrombosis in PNH; however, most PNH patients with a history of thrombosis can safely discontinue anticoagulation after therapeutic complement inhibition. Median survival before complement inhibitors was roughly 15 years, and patients often suffered from extreme fatigue, headaches, painful abdominal crises due to smooth muscle dystonias, and the need for frequent blood transfusions.13
Pros and cons of C5 inhibition
C5 inhibition with eculizumab or its derivative, ravulizumab, is the current standard to treat PNH. Ravulizumab is preferable to eculizumab due to its longer half-life that allows for intravenous dosing every 8 weeks.14,15 C5 inhibitors improve quality of life, decrease the need for blood transfusions, and prevent thrombosis,16 the leading cause of death from PNH. The drugs are well tolerated. The major adverse event is an increased risk for neisserial infections, roughly 0.5% risk per year on drug. C5 inhibitors abolish the need for transfusions in up to 80% of patients, but most PNH patients on C5 inhibitors have extravascular hemolysis and mild to moderate anemia.17,18 Patients who are asymptomatic or mildly symptomatic need no additional therapy; however, up to a third of PNH patients treated with C5 inhibitors remain transfusion dependent or have bothersome symptoms (fatigue, dyspnea) from their anemia and could benefit from a proximal complement inhibitor.
Pegcetacoplan and PNH
The 3 pathways of complement activation converge at the level of C3 cleavage. Opsonization of PNH erythrocytes with C3 fragments and their subsequent phagocytosis in the liver and spleen accounts for the extravascular hemolysis observed in PNH patients on C5 inhibition.19 Proximal complement inhibitors are in development to address persistent symptomatic anemia resulting from C3-mediated extravascular hemolysis (Figure 1).8,20,21 Pegcetacoplan was the first proximal complement inhibitor to receive US Food and Drug Administration approval in May 2021 for frontline treatment of PNH and European Medicines Agency approval in December 2021 for PNH patients who remain anemic despite ≥3 months of treatment with a C5 inhibitor. Administered as a twice-weekly subcutaneous infusion, pegcetacoplan is a polyethylene glycol (PEG)ylated peptide that binds to and inhibits C3 and its cleavage fragment C3b, thus attenuating both C3-mediated extravascular hemolysis and intravascular hemolysis from downstream, terminal complement activation.22
Structure and function of pegcetacoplan. (A) Schematic of pegcetacoplan structure. Pegcetacoplan is a PEGylated peptide of the compstatin family composed of 2 identical cyclic peptides covalently bound to a PEG molecule. Compstatin and its analogs bind in a shallow groove in the C-terminal fragment of the complement C3 β-chain. There is extensive interaction between C3 and compstatin. The green circles represent hydrophobic interactions and hydrogen bonding between compstatin derivatives and C3.23 (B) Complement pathway inhibitors approved for clinical use or in trial for PNH and their sites of inhibition. Pegcetacoplan and other compstatin family derivatives sterically inhibit the interaction between C3 and the C3 convertase through binding to C3 or C3b. Iptacopan (factor B inhibitor), danicopan (factor D inhibitor), and BCX9930 (factor D inhibitor) are oral proximal complement inhibitors in phase 2 and 3 clinical trials for PNH, both as monotherapy and in combination with a C5 inhibitor.
Structure and function of pegcetacoplan. (A) Schematic of pegcetacoplan structure. Pegcetacoplan is a PEGylated peptide of the compstatin family composed of 2 identical cyclic peptides covalently bound to a PEG molecule. Compstatin and its analogs bind in a shallow groove in the C-terminal fragment of the complement C3 β-chain. There is extensive interaction between C3 and compstatin. The green circles represent hydrophobic interactions and hydrogen bonding between compstatin derivatives and C3.23 (B) Complement pathway inhibitors approved for clinical use or in trial for PNH and their sites of inhibition. Pegcetacoplan and other compstatin family derivatives sterically inhibit the interaction between C3 and the C3 convertase through binding to C3 or C3b. Iptacopan (factor B inhibitor), danicopan (factor D inhibitor), and BCX9930 (factor D inhibitor) are oral proximal complement inhibitors in phase 2 and 3 clinical trials for PNH, both as monotherapy and in combination with a C5 inhibitor.
Pegcetacoplan (APL-2) is a member of the compstatin family of cyclic peptides, which selectively bind human and nonhuman primate C3 and C3b and prevent interaction with the C3 convertase through steric hindrance.24 The initial molecule of this class was discovered in 1996 through a phage-displayed peptide library screen for C3b-binding.25 Subsequent derivatives with enhanced inhibitory activity (1MeW, Cp40) and the addition of polyethylene glycol moieties yielded peptide drugs with longer half-life properties.22 In preclinical studies in PNH, these peptidic C3 inhibitors (Cp40) were found to prevent hemolysis and C3 fragment opsonization of PNH erythrocytes in vitro in a dose-dependent manner.26 Further, pharmacokinetic studies in nonhuman primates showed that sustained, systemic therapeutic concentrations could be achieved with subcutaneous infusions. In 2020, the open-label, phase 1b PHAROAH trial confirmed that pegcetacoplan concentration accumulated with repeated subcutaneous dosing and reached steady-state levels after 6 to 8 weeks of administration.27 Pegcetacoplan increased hemoglobin levels and decreased reticulocyte count and total bilirubin in 6 PNH patients who were anemic on eculizumab treatment alone. Four patients were successfully transitioned to pegcetacoplan monotherapy and maintained transfusion independence. Further, in this small study, pegcetacoplan was well tolerated with the majority of treatment-related adverse events due to infusion site reactions, and no adverse events led to drug discontinuation.
In the recent phase 3 PEGASUS trial comparing pegcetacoplan to eculizumab in patients ≥18 years old with PNH and anemia despite eculizumab therapy (hemoglobin <10.5 g/dL), Hillmen et al demonstrated a significant improvement in hemoglobin levels.8 After a 4-week run-in period when all patients received pegcetacoplan (1080 mg subcutaneously twice per week) in addition to eculizumab, patients were randomized to receive pegcetacoplan (41 patients) or eculizumab (39 patients) monotherapy. During the 16-week randomization period, patients receiving pegcetacoplan had an average increase in hemoglobin from baseline of +2.3 g/dL vs −1.5 g/dL in the eculizumab group. A statistically significant reduction in transfusion requirements was observed in the pegcetacoplan group (85% transfusion independence in patients receiving pegcetacoplan vs 15% on eculizumab). Noninferiority was observed for change in absolute reticulocyte count but not for change in lactate dehydrogenase (LDH), suggestive of the effect of pegcetacoplan on extravascular hemolysis. Fatigue metrics improved in the patients receiving pegcetacoplan. Common adverse events associated with pegcetacoplan as compared with eculizumab included injection site reactions (37% vs 3%), diarrhea (22% vs 3%), and infections (29% vs 26%). Three patients in the pegcetacoplan group discontinued therapy during the 16-week study period due to breakthrough hemolysis with significantly elevated LDH greater than 3 times the upper limit of normal (3× ULN). There were no reported thromboembolic events, cases of meningitis, or deaths. In the 48-week extension study including the randomized control and open-label periods (77 patients with crossover of eculizumab patients to pegcetacoplan after week 16), patients on pegcetacoplan monotherapy maintained improved hemoglobin levels, and patients switched to pegcetacoplan demonstrated similar improvements in hemoglobin.28 During the 48 weeks, >70% of patients on pegcetacoplan maintained transfusion independence. However, 24% of patients experienced physician-reported hemolysis, and 6 patients discontinued the drug due to hemolytic events (12 total patients discontinued the drug). Pegcetacoplan treatment likely results in an increased PNH red cell clone and, consequently, breakthrough hemolysis may be more severe, with LDH ∼10× to 15× ULN. As highlighted by correspondence following the PEGASUS trial, the severity of breakthrough hemolysis in some patients on pegcetacoplan differs significantly from that observed in patients on C5 inhibition and more closely resembles the intravascular hemolysis in untreated PNH patients.29
In PNH patients with persistent, symptomatic anemia due to extravascular hemolysis on C5 inhibition, pegcetacoplan represents a practice-changing advance. Additionally, for the small subgroup of PNH patients with genetic variants in C5, pegcetacoplan may be effective.30 Route of administration, large volume of subcutaneous injection (20 mL per dose), need for refrigeration, and the short half-life are limitations of pegcetacoplan. Although clinical trial data and early experience with pegcetacoplan monotherapy is promising, long-term follow-up of efficacy, specifically in terms of breakthrough hemolysis, as well as safety and tolerability are needed. Pegcetacoplan is presumed to reduce thrombotic events based on its mechanism of action and extrapolation of experience from C5 inhibition; however, data on the efficacy of pegcetacoplan in thrombosis risk reduction is overall lacking. Further, the time to reach steady-state concentration (4-6 weeks) may limit its use in the setting of acute thrombosis. There is also a lack of data on the safety and efficacy of pegcetacoplan in pregnancy, in which case C5 inhibition is preferred. In addition, results from clinical trials of other proximal complement inhibitors, either as monotherapy or in conjunction with a C5 inhibitor, appear favorable and will likely continue to expand treatment options for PNH patients in the coming years. Iptacopan (factor B inhibitor), danicopan (factor D inhibitor) and BCX9930 (factor D inhibitor) are oral proximal complement inhibitors in phase 2 and 3 clinical trials for PNH but not yet approved.
Sequencing complement inhibitors in PNH
We report our approach to the use of clinically available complement inhibitors in PNH; these recommendations are based on expert opinion given the paucity of head-to-head trials of pegcetacoplan in the frontline setting. C5 inhibition, preferably with ravulizumab, is our preferred frontline therapy for PNH (Table 1). Onset of action is immediate, every-8-weeks dosing is convenient, and there is almost 2 decades of experience with C5 inhibitors. Pegcetacoplan is administered by subcutaneous infusion (over 30-60 minutes) twice a week, must be stored refrigerated, and takes several weeks to reach steady state. Further, the phase 3 trial and extension study of pegcetacoplan suggest a significant risk of severe breakthrough hemolysis. There are not yet standard guidelines for management of this potentially life-threatening complication. In the case of breakthrough hemolysis, authors from the PEGASUS trial recommend consideration of immediate red cell transfusion, pegcetacoplan dose adjustment, or short-term use of eculizumab as well as treatment of any underlying complement amplifying conditions.29
Route of administration and standard dosing recommendations for approved complement inhibitors in adult patients with PNH
| Drug . | Target . | Route . | Loading dose . | Maintenance dose . | Frequency . |
|---|---|---|---|---|---|
| Eculizumab | C5 | Intravenous over 1 h | 600 mg weekly ×4 doses; maintenance dosing starting week 5 | 900 mg | Every 2 wk |
| Ravulizumab | C5 | Intravenous over 1 h | ≥40 to <60 kg: 2400 mg ≥60 to <100 kg: 2700 mg ≥100 kg: 3000 mg; start maintenance dosing 2 wk later | ≥40 to <60 kg: 3000 mg ≥60 to <100 kg: 3300 mg ≥100 kg: 3600 mg | Every 8 wk |
| Pegcetacoplan | C3 | Subcutaneous infusion over 30-60 min | Overlap with C5 inhibitor for 4 wk | 1080 mg | Twice weekly; every 3 d for LDH >2× ULN |
| Drug . | Target . | Route . | Loading dose . | Maintenance dose . | Frequency . |
|---|---|---|---|---|---|
| Eculizumab | C5 | Intravenous over 1 h | 600 mg weekly ×4 doses; maintenance dosing starting week 5 | 900 mg | Every 2 wk |
| Ravulizumab | C5 | Intravenous over 1 h | ≥40 to <60 kg: 2400 mg ≥60 to <100 kg: 2700 mg ≥100 kg: 3000 mg; start maintenance dosing 2 wk later | ≥40 to <60 kg: 3000 mg ≥60 to <100 kg: 3300 mg ≥100 kg: 3600 mg | Every 8 wk |
| Pegcetacoplan | C3 | Subcutaneous infusion over 30-60 min | Overlap with C5 inhibitor for 4 wk | 1080 mg | Twice weekly; every 3 d for LDH >2× ULN |
Pegcetacoplan should be considered in patients who remain transfusion-dependent or have symptomatic anemia attributable to extravascular hemolysis despite a 3-month or more trial of C5 inhibition. We consider extravascular hemolysis as the likely culprit for persistent anemia when LDH ≤1.5× ULN and/or the CH50 is completely blocked, as elevations in these are more suggestive of intravascular hemolysis and incomplete terminal complement inhibition, and the reticulocyte count is elevated (≥100 × 109 cells per liter).31,32 Practitioners must be mindful of other causes for anemia in PNH, such as bone marrow failure, nutritional deficiencies, relative erythropoietin deficiency, bleeding, hypersplenism, and presence of transfusion-related alloantibodies.31 In these cases, proximal complement inhibition is unlikely to be efficacious. As we gain more experience with pegcetacoplan, it may be reasonable to start pegcetacoplan as frontline therapy in select patients.
Future directions
Multiple trials of pegcetacoplan in both PNH and for other indications are ongoing. Examples of potentially practice-changing trials in PNH include use of pegcetacoplan in the frontline setting for patients not on complement inhibition (clinicaltrials.gov #NCT04085601) and pegcetacoplan for pediatric patients (#NCT04901936). In terms of other complement-mediated diseases, C3 inhibition may have wide-ranging therapeutic implications. Within hematology, there are active trials of pegcetacoplan in cold agglutinin disease (#NCT05096403), hematopoietic stem cell transplant–associated thrombotic microangiopathy (#NCT05148299), and autoimmune hemolytic anemia (#NCT03226678). Several trials of intravitreal pegcetacoplan for geographic atrophy secondary to age-related macular degeneration are underway (#NCT03525600, #NCT03525613, and #NCT04770545). In neurology, complement has been implicated in the pathogenesis of amyotrophic lateral sclerosis, and a phase 2 trial of pegcetacoplan is recruiting (#NCT04579666).33,34 Additionally, there are active clinical trials of pegcetacoplan in glomerulopathies (#NCT03453619), C3 glomerulopathy or immune-complex membranoproliferative glomerulonephritis (#NCT05067127), and, specifically, recurrence posttransplant (#NCT04572854). Other compstatin derivatives are also under clinical investigation. A phase 2a trial of intragingival injections of the third-generation Cp40-based compstatin analog AMY-101 in periodontal disease showed reduction in markers of gingival inflammation.35 Recently, in a small study of patients with severe COVID-19, AMY-101 showed improvements in inflammatory markers and pulmonary function.36 Studies of new Cp40 analogs demonstrated improved solubility, increased half-life, and slower drug clearance in nonhuman primates, with potential to reduce dosing frequency and allow delivery of higher doses.37
Authorship
Contribution: R.A.B. designed and wrote the manuscript and G.F.G. created the figure and wrote the manuscript.
Conflict-of-interest disclosure: R.A.B. receives research funding from Alexion. G.F.G. declares no competing financial interests.
Correspondence: Robert A. Brodsky, Division of Hematology, School of Medicine, Johns Hopkins University, 720 Rutland Ave, Ross Research Building, Room 1025, Baltimore, MD 21205; e-mail: brodsro@jhmi.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal