In this issue of Blood, Mei et al describe the results of treating relapsed or refractory (RR) Hodgkin lymphoma (HL) with the anti-programmed cell death protein 1 (PD-1) antibody nivolumab, that show a high response rate to antibody monotherapy.1 A response-adapted approach was used to determine whether patients proceeded to high-dose consolidation therapy (HDCT) after treatment with antibody alone or received additional salvage chemotherapy. The proportion of patients who remained free from recurrence was high, so this may become an attractive option for second-line therapy.
Although modern first-line treatments for classical HL are successful in eradicating the disease in ∼80% of patients,2 there remains a need for effective treatment for those in whom initial therapy is not successful. The treatment strategy for RR HL has been largely unchanged since it was shown more than 20 years ago that salvage chemotherapy followed by HDCT with autologous stem cell transplantation (HDCT/ASCT) can be curative in a majority of patients.3 A variety of cytotoxic salvage regimens have been developed, but so far, there has been no compelling evidence that any of them is superior in terms of disease control.4-6 These regimens produce objective response rates of ∼80%, and progression-free survival (PFS) rates after HDCT of 60% to 70% at 5 years, a figure that is higher for patients who proceed to HDCT when they are in metabolic complete remission (CR) according to 18F‐fluorodeoxyglucose positron emission tomography (FDG-PET) scanning.7
The development of antibody-targeted therapies for HL holds the promise of more selective treatment, which may provide similar or higher levels of efficacy, potentially with reduced toxicity. The antibody-drug conjugate brentuximab vedotin (BV), which directs the microtubule disrupting agent monomethyl auristatin E to cells expressing the CD30 antigen, has been used in combination with chemotherapy salvage treatment.8 One study used a sequential approach, giving BV alone as initial salvage therapy. The response was assessed by PET, and treatment proceeded directly to HDCT/ASCT in the case of metabolic CR or was escalated by the addition of chemotherapy before HDCT.9 Among 46 patients treated in this way, 12 proceeded to HDCT/ASCT after BV alone, and another 32 also received chemotherapy after a positive interim PET scan. For the 44 patients who proceeded to HDCT/ASCT, the event-free survival (EFS) rate at 2 years was 80%. Among those who became PET negative after being treated with BV alone, the 2-year EFS was 92%. The attraction of this approach is that it averts the substantial toxicity of conventional salvage chemotherapy for approximately a quarter of those treated.
Another option for antibody targeting in HL is the use of anti-PD-1 antibodies. The ligands for PD-1 are present on Hodgkin Reed-Sternberg (HRS) cells as well as tumor-associated macrophages (TAMs), and the antibodies seem to act by interrupting the signaling between them and the infiltrating T cells that express PD-1. This is in contrast to the mechanism of action suggested in solid tumor oncology, in which anti-PD-1 is thought to reverse immune checkpoint blockade of cytotoxic T-cell proliferation, thereby restoring effective responses against tumor neoantigens. The very rapid responses seen in HL to anti-PD-1 therapy are more compatible with a direct effect on cell survival than with generation of an adaptive immune response; this was supported by a study of sequential biopsies in patients treated with nivolumab alone or in combination with chemotherapy.10 HRS cells and TAMs were rapidly depleted within days of starting treatment, and there was some reduction of type 1 regulatory T cells but no apparent expansion of cytotoxic T cells.
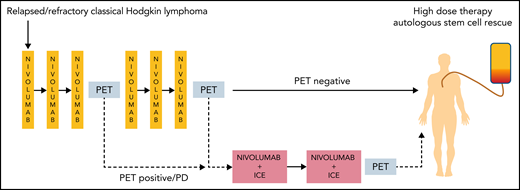
The study by Mei et al took a PET-adapted approach for salvage therapy similar to that previously tested with BV. Patients started treatment with nivolumab alone given once every 2 weeks, and responses were assessed by PET scans (see figure). Treatment proceeded directly to HDCT/ASCT after 6 doses for those in metabolic CR. For those who remained positive by PET scanning or progressed with nivolumab alone, chemotherapy with nivolumab plus ifosfamide, carboplatin, and etoposide (NICE) was added for 2 cycles. Among 43 patients enrolled, 3 did not complete 3 doses of nivolumab because of autoimmune toxicity, and 9 went on to receive NICE. Of 30 patients who reached metabolic CR after nivolumab alone, 26 proceeded to HDCT/ASCT, as did another 7 patients who required NICE. The levels of stem cell mobilization and engraftment after high-dose chemotherapy were in line with expected outcomes. The overall response rate to nivolumab alone was 34 (81%) of 42, with 30 (71%) CRs. In keeping with the hypothesis that anti-PD-1 antibodies work by interrupting intercellular signals rather than by inducing adaptive immunity, analysis of the cellular composition of biopsies taken before treatment showed lower numbers of PD-1–positive CD8+ T cells and CD163+ TAMs in patients who achieved a CR, possibly because the microenvironmental survival signals were easier to block in these cases. The 2-year PFS rate was 73% among the whole cohort of 43 patients. Those who proceeded to HDCT/ASCT after nivolumab alone had a 2-year PFS of 94%, but it should be noted that 22 (69%) of them also received posttransplant maintenance with BV, nivolumab or, in most cases, both agents. Whether this was necessary will need to be explored, and the minority of patients who did not receive post-transplant therapy did not experience any treatment failures.
Adaptive treatment scheme, using FDG-PET to determine whether patients proceed to HDCT after nivolumab alone or combination chemoimmunotherapy. PD, progressive disease.
Adaptive treatment scheme, using FDG-PET to determine whether patients proceed to HDCT after nivolumab alone or combination chemoimmunotherapy. PD, progressive disease.
Overall, these results are comparable to those seen in the study that used BV as monotherapy9 in a broadly similar cohort of patients, although the proportion of patients who were able to proceed directly to HDCT after nivolumab was higher than that after BV. The potential to spare 70% of patients salvage chemotherapy before HDCT/ASCT with this approach is clearly attractive. The initial autoimmune toxicities after nivolumab (which led to the withdrawal of 3 patients) are a potential concern, but the toxicity was otherwise limited, and no other grade 3 events were recorded after antibody monotherapy.
In the Mei et al study, only 4 patients had been previously treated with BV, but as first-line treatments evolve in response to the data emerging from clinical trials, it is likely that the proportion of patients pre-exposed to BV will increase, potentially making the option of anti-PD-1 antibodies for second-line therapy preferable.
Conflict-of-interest disclosure: P.W.M.J. has received honoraria from Millennium Pharmaceuticals, Takeda, and Bristol Myers Squibb.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal