Key Points
Children with a history of CVC-associated thrombosis are at increased risk for recurrent VTE.
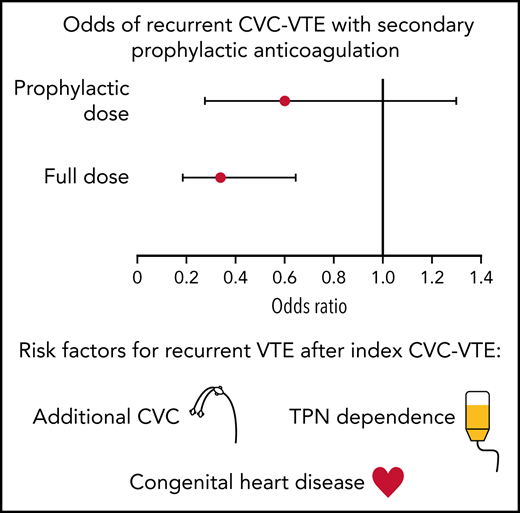
With subsequent CVC placement, secondary prophylaxis with full-dose anticoagulation was associated with a 65% reduction in thromboembolism.
Abstract
Central venous catheters (CVC) are the most significant risk factor for pediatric venous thromboembolism (VTE). After an index CVC-associated VTE (CVC-VTE), the role of secondary prophylaxis for subsequent CVC placement is uncertain. Aims of this single-center retrospective study were to evaluate the efficacy of secondary prophylaxis for patients with a prior CVC-VTE and identify risk factors associated with recurrent VTE in patients less than 19 years with an index CVC-VTE between 2003 and 2013. Data collection included clinical and demographic factors, subsequent CVC placement, secondary prophylaxis strategy, recurrent VTE, and bleeding. Risk factors for recurrence and effectiveness of secondary prophylaxis were evaluated using survival and binomial models. Among 373 patients with an index CVC-VTE, 239 (64.1%) had subsequent CVC placement; 17.4% (65/373) of patients had recurrent VTE, of which 90.8% (59/65) were CVC-associated. On multivariable survival analysis, each additional CVC (hazards ratio [HR] 12.00; 95% confidence interval [CI] 2.78-51.91), congenital heart disease (HR 3.70; 95% CI 1.97-6.95), and total parenteral nutrition dependence (HR 4.02; 95% CI 2.23-7.28) were associated with an increased hazard of recurrence. Full dose anticoagulation for secondary prophylaxis was associated with decreased odds of recurrent CVC-VTE (odds ratio [OR] 0.35; 95% CI 0.19-0.65) but not prophylactic dosing (OR 0.61; 95% CI 0.28-1.30). Only 1.3% of CVCs experienced major bleeding with prophylactic or full-dose anticoagulation. In summary, children with CVC-VTE are at increased risk for recurrent VTE. Secondary prophylaxis with full-dose anticoagulation was associated with a 65% reduction in odds of thrombotic events.
Introduction
Catheter-related thrombosis is a major problem in pediatric tertiary care hospitals.1,2 A 2009 study of pediatric hospital-acquired venous thromboembolism (VTE) by Raffini et al estimated a 70% increase in the annual rate of VTE in the years 2001 to 2007.2 The presence of a central venous catheter (CVC) remains the single most common risk factor for the development of VTE in children.3,4 The Children’s Hospital Acquired Thrombosis consortium demonstrated that 80% of hospital-acquired VTE in their registry were associated with a CVC.5 The majority of pediatric patients with VTE have multiple comorbidities in addition to a CVC that contribute to their VTE risk.3,6 As the medical care of chronically ill pediatric patients becomes increasingly complex, it is reasonable to assume that the incidence of pediatric VTE will continue to rise without directed prevention interventions.
Children with chronic medical illnesses often require multiple CVCs complicated by recurrent thrombotic events. There is a lack of data with respect to risk factors for developing recurrent thrombotic events with subsequent CVC placement and if there is a role for secondary prophylaxis. The 2012 American College of Chest Physicians clinical practice guidelines for antithrombotic therapy in neonates and children only address prophylactic anticoagulation if the original catheter that provoked the thrombotic event remains in place after a complete course of therapeutic anticoagulation.7 In this situation, there is a weak grade 2C recommendation to continue reduced intensity (prophylactic dose) anticoagulation until the CVC is removed. The 2018 American Society of Hematology guidelines for the management of VTE in pediatric patients do not address secondary CVC prophylaxis.8 Currently, physicians are forced to make these challenging clinical decisions without the support of data or formalized recommendations regarding the role of anticoagulation with additional CVC placements, resulting in practice variation.9
In our institution, we initially recommended prophylactic dosing of anticoagulation in patients with a prior history of CVC-VTE who required another catheter. However, after clinically noting many breakthrough thrombotic events despite prophylactic dosing, we modified our clinical practice to full-dose anticoagulation in patients with a prior history of CVC-VTE who required another catheter. In addition, provider preference and a patient’s bleeding risk impacted individual prophylactic strategies.
The objective of this study was to evaluate the efficacy of secondary prophylaxis in the setting of subsequent CVC placement for patients with a prior history of CVC-VTE and to identify risk factors for recurrent CVC-VTE.
Methods
This study was approved by the Institutional Review Board at the Children’s Hospital of Philadelphia (CHOP).
Data source
Several existing data sources were used for this single-center, retrospective cohort study, including an existing clinical thrombosis database within the CHOP Division of Hematology as well as an inpatient database for hospital-acquired VTE. Data collection used electronic medical records.
Data collection and study definitions
Subjects were included if they were younger than 19 years of age at the time of their first radiologically confirmed index CVC-VTE event between 1 January 2003 and 31 December 2013. Data were collected on all subjects from the time of their index CVC-VTE until their study-terminating event. A study-terminating event was defined as death, recurrent CVC-VTE, transfer of care to another institution, or the designated study end date of 30 September 2014.
Index CVC-VTE event
CVC-VTE events in this study were identified as part of routine clinical care. Routine screening imaging (ie, ultrasound) was not performed, although VTE captured in our study may have been detected on an imaging study performed for another indication. Data elements recorded for the index CVC-VTE event included: demographics, index CVC-VTE details (date, location of VTE, type of CVC, location of CVC, clinical symptoms), underlying medical conditions, identified prothrombotic conditions if assessed, and the anticoagulation treatment regimen. Acute prothrombotic conditions included recent surgery (<30 days), active infection (sepsis, bacteremia, soft-tissue bacterial infection, osteomyelitis, CVC/shunt infection), protein-losing process (nephrotic syndrome, chylous pleural effusion), or prothrombotic medication (estrogen-containing oral contraceptive pills or l-asparaginase therapy). Congenital thrombophilia was diagnosed by a hematologist (after repeated low age-dependent values for anticoagulant protein deficiencies) and defined as: antithrombin deficiency, protein C deficiency, protein S deficiency, factor V Leiden mutation, and/or prothrombin gene mutation.
Subsequent CVC placement
We collected the following details on all subsequent CVCs placed after the index CVC-VTE until a study-terminating event: date of placement and removal, type of CVC, location, and use of anticoagulation (drug and intensity) for secondary prophylaxis. For some patients, anticoagulation was indicated for reasons other than the presence of another CVC (ie, extracorporeal membrane oxygenation). The anticoagulation was classified as full dose or prophylactic dose. Full-dose anticoagulation included warfarin (goal international normalized ratio [INR] 2-3), enoxaparin (goal anti-Xa 0.5-1), heparin (partial thromboplastin time [PTT] 60 to 80 seconds), and bivalirudin (dilute thrombin time 60 to 90 seconds). Prophylactic dose anticoagulation included warfarin (goal INR 1.5-2), enoxaparin (0.5 mg/kg per dose every 12 hours without anti-Xa monitoring), and low dose heparin (10 to 15 units/kg per hour with no monitoring). Subjects who received both full and prophylactic dosing of anticoagulation while a CVC was in place were analyzed as receiving both.
Recurrent CVC-VTE event
A recurrent thrombotic event was defined as a VTE that was diagnosed at least 7 days after the index thrombotic event. These were identified by a comprehensive chart review of every outpatient, emergency department, and inpatient encounter. Events were separated into CVC-associated recurrent events and non–CVC-associated recurrent events. A recurrent CVC-VTE in the same location as the index CVC-VTE was only included if there was clear radiologic evidence of resolution prior to the second event. Anticoagulation for secondary prophylaxis was categorized as full dose, prophylactic dose, or no anticoagulation based on the regimen at the time of recurrence.
Bleeding events
To assess the harm associated with the secondary prophylaxis treatment strategy, the risk of bleeding during secondary prophylaxis, and not with anticoagulation used for the treatment of the index VTE, was captured. Bleeding events were identified by review of every outpatient, emergency department, and inpatient encounter during the secondary prophylaxis time period. The secondary prophylaxis time period was defined as the time period after 3 months of treatment of the index VTE and continued until a study terminating event. Major bleeding and clinically relevant nonmajor (CRNM) bleeding were defined utilizing the established recommended pediatric outcome definitions.10 The analysis was completed as a bleeding event per CVC.
Statistical analysis
Study variables were summarized using frequencies and percentages for categorical variables. Time until recurrent thrombosis was analyzed with Cox proportional hazards models. Exposure variables considered were age group, sex, race, total number CVCs placed, and chronic and acute medical conditions. Univariate analysis was performed, and statistically significant and clinically relevant variables were included in a multivariable model. Hazard ratios (HR) with 95% confidence intervals and P values are presented from these models. Kaplan Meier survival curves were developed for select exposure variables.
Given the significance of additional CVC placement in the primary analysis, we performed a secondary analysis using CVCs as the point of analysis. A generalized estimating equations binomial-logit model with exchangeable correlation assumed for CVCs from the same patient was used for this analysis. In addition to the exposure variables used in the primary analysis, anticoagulation dose intensity and CVC type were included as those varied for each additional CVC placed. Similarly, models were run as univariate, and then based on these results, statistically significant and clinically relevant variables were included in the multivariable model. Odds ratios (OR) with 95% confidence intervals and P values are presented from these models. An α level of 0.05 was considered for significance. All analyses were performed using SAS version 9.4 software (SAS Institute, Cary, NC) or R version 4.0.3 software (R Foundation for Statistical Computing, Vienna, Austria).
Results
Between 2003 and 2013, 373 unique subjects had an index CVC-VTE. The median follow-up time was 688 days (interquartile range [IQR] 158-1271). Forty-seven patients (12.6% of the total cohort) left before the study period ended. Patient demographics and distribution of VTE risk factors are summarized in Table 1. Patients younger than 1 year of age accounted for 59% (218/373) of the study cohort. The index VTE was associated with a peripherally inserted central catheter (PICC) in the majority (59%, 219/373). Eighteen percent (68/373) of subjects had congenital heart disease, and 14% (54/373) were TPN dependent.
Summary statistics of total study cohort (n = 373)
| Patient demographics . | Entire cohort (n = 373), % . |
|---|---|
| Age at study entry (y) | |
| <1 | 218 (59) |
| 1-5 | 78 (24) |
| 5-12 | 48 (11) |
| >12 | 29 (7) |
| Sex | |
| Male | 203 (54) |
| Female | 170 (46) |
| Race | |
| White | 182 (49) |
| Black | 95 (25) |
| Asian | 16 (4) |
| Other | 80 (21) |
| Follow-up time (d) | |
| Median (IQR 25-75) | 688 (158-1271) |
| Total additional CVCs | |
| 0 | 134 (36) |
| 1 | 102 (27) |
| 2 | 63 (17) |
| 3 | 34 (9) |
| 4 or more | 40 (11) |
| Chronic medical condition | |
| Congenital heart disease | 68 (18) |
| Total parenteral nutrition dependence | 54 (14) |
| Cancer | 30 (8) |
| Renal disease | 23 (6) |
| Hyperinsulinemia | 24 (6) |
| Cystic fibrosis | 6 (2) |
| Autoimmune disease | 4 (1) |
| Gastrointestinal autoimmune disease | 10 (3) |
| Sickle cell disease | 8 (2) |
| Other | 213 (57) |
| None | 45 (12) |
| Congenital thrombophilia* | |
| Present | 36 (10) |
| Absent | 203 (54) |
| Not Assessed | 134 (36) |
| Acute prothrombotic condition | |
| Recent surgery (<30 d) | 78 (21) |
| Active infection | 71 (19) |
| Protein-losing process | 11 (3) |
| Prothrombotic medication | 11 (3) |
| Index CVC type | |
| Peripherally inserted central catheter | 219 (59) |
| Tunneled catheter | 19 (5) |
| Nontunneled catheter | 135 (3) |
| Patient demographics . | Entire cohort (n = 373), % . |
|---|---|
| Age at study entry (y) | |
| <1 | 218 (59) |
| 1-5 | 78 (24) |
| 5-12 | 48 (11) |
| >12 | 29 (7) |
| Sex | |
| Male | 203 (54) |
| Female | 170 (46) |
| Race | |
| White | 182 (49) |
| Black | 95 (25) |
| Asian | 16 (4) |
| Other | 80 (21) |
| Follow-up time (d) | |
| Median (IQR 25-75) | 688 (158-1271) |
| Total additional CVCs | |
| 0 | 134 (36) |
| 1 | 102 (27) |
| 2 | 63 (17) |
| 3 | 34 (9) |
| 4 or more | 40 (11) |
| Chronic medical condition | |
| Congenital heart disease | 68 (18) |
| Total parenteral nutrition dependence | 54 (14) |
| Cancer | 30 (8) |
| Renal disease | 23 (6) |
| Hyperinsulinemia | 24 (6) |
| Cystic fibrosis | 6 (2) |
| Autoimmune disease | 4 (1) |
| Gastrointestinal autoimmune disease | 10 (3) |
| Sickle cell disease | 8 (2) |
| Other | 213 (57) |
| None | 45 (12) |
| Congenital thrombophilia* | |
| Present | 36 (10) |
| Absent | 203 (54) |
| Not Assessed | 134 (36) |
| Acute prothrombotic condition | |
| Recent surgery (<30 d) | 78 (21) |
| Active infection | 71 (19) |
| Protein-losing process | 11 (3) |
| Prothrombotic medication | 11 (3) |
| Index CVC type | |
| Peripherally inserted central catheter | 219 (59) |
| Tunneled catheter | 19 (5) |
| Nontunneled catheter | 135 (3) |
Congenital thrombophilia defined as factor V Leiden mutation, antithrombin deficiency, protein C deficiency, protein S deficiency, and/or prothrombin gene mutation.
64.1% (239/373) of subjects had a total of 550 additional CVCs placed, with a median of 2 additional CVCs per subject during the study period (IQR 1-3). The median time from index CVC-VTE to placement of a subsequent CVC was 4 days (IQR 0-25). Of the 550 additional CVCs, 64% (354/549) were PICCs, 15% (84/550) tunneled lines, and 20% (112/550) nontunneled lines. Prophylaxis strategies included only full-dose anticoagulation in 53% (289/550) of lines, only prophylactic dose in 14% (78/550), both full and prophylactic dose in 3% (15/550), and no anticoagulation in 31% (168/550).
Of the 373 patients with index CVC-VTE, 65 (17.4%) had recurrent thrombotic events, 59 of which (90.8%) were associated with a CVC. The median time to recurrence of CVC-VTE from index event was 90.5 days (IQR 30-178). The median time to a recurrent VTE after subsequent CVC placement was 7.5 days (IQR 4-20). Of the recurrent CVC-VTE, 84.7% (50/59) were related to a new CVC in a new location, while 13.6% (8/59) were related to a new CVC placed in the same location as the previously resolved clot, and 1.7% (1/59) recurred around the same port-a-catheter that was present during the index event. 9.2% (6/65) of the recurrent VTE were not CVC associated (Table 2). The median time from the index CVC-VTE to the non-CVC associated recurrent event was 136.5 days (IQR 57-380).
Non-CVC-associated recurrent thrombotic events
| Chronic medical condition (age at recurrence) . | Initial site of thrombosis . | Recurrent wite of thrombosis . | VTE risk factors and anticoagulation . | Time to recurrent event (d) . | Patient location of recurrent clot . |
|---|---|---|---|---|---|
| Congenital heart disease (17 y) | Right internal jugular | Left internal jugular | Sepsis. Not on anticoagulation. | 380 | Pediatric ICU |
| Total parenteral nutrition dependence (16 y) | Right axillary vein | Left portal vein | Recent surgery (auto islet cell transplant 2 d prior, occurred via portal vein), immobility. On full-dose heparin. | 716 | Pediatric ICU |
| Biliary atresia (4 mo) | Right superficial femoral, common femoral, external iliac, and popliteal veins | Portal vein | Recent surgery (IR liver biopsy 3 d prior), subdural hemorrhage (not associated with anticoagulation). Not on anticoagulation. | 9 | Inpatient |
| Congenital heart disease (23 mo) | Left common femoral vein | Fontan fenestration | Recent surgery (15 d prior lateral tunnel fenestrated Fontan), staph bacteremia, viral gastroenteritis. Off anticoagulation due to a subdural hemorrhage. | 57 | Cardiac ICU |
| Congenital heart disease (12 mo) | Left brachial vein | Left external iliac | Respiratory failure, status post congenital twin separation 5 mo prior. Off anticoagulation due to major bleeding. | 118 | Cardiac ICU |
| Hypoxic ischemic encephalopathy (8 y) | Right common femoral and external iliac veins |
| Active GI bleeding, heterozygous Factor V Leiden. Off anticoagulation due to GI bleeding. | 155 | Pediatric ICU |
| Chronic medical condition (age at recurrence) . | Initial site of thrombosis . | Recurrent wite of thrombosis . | VTE risk factors and anticoagulation . | Time to recurrent event (d) . | Patient location of recurrent clot . |
|---|---|---|---|---|---|
| Congenital heart disease (17 y) | Right internal jugular | Left internal jugular | Sepsis. Not on anticoagulation. | 380 | Pediatric ICU |
| Total parenteral nutrition dependence (16 y) | Right axillary vein | Left portal vein | Recent surgery (auto islet cell transplant 2 d prior, occurred via portal vein), immobility. On full-dose heparin. | 716 | Pediatric ICU |
| Biliary atresia (4 mo) | Right superficial femoral, common femoral, external iliac, and popliteal veins | Portal vein | Recent surgery (IR liver biopsy 3 d prior), subdural hemorrhage (not associated with anticoagulation). Not on anticoagulation. | 9 | Inpatient |
| Congenital heart disease (23 mo) | Left common femoral vein | Fontan fenestration | Recent surgery (15 d prior lateral tunnel fenestrated Fontan), staph bacteremia, viral gastroenteritis. Off anticoagulation due to a subdural hemorrhage. | 57 | Cardiac ICU |
| Congenital heart disease (12 mo) | Left brachial vein | Left external iliac | Respiratory failure, status post congenital twin separation 5 mo prior. Off anticoagulation due to major bleeding. | 118 | Cardiac ICU |
| Hypoxic ischemic encephalopathy (8 y) | Right common femoral and external iliac veins |
| Active GI bleeding, heterozygous Factor V Leiden. Off anticoagulation due to GI bleeding. | 155 | Pediatric ICU |
GI, gastrointestinal; ICU, intensive care unit; IR, interventional radiology; VTE, venous thromboembolism.
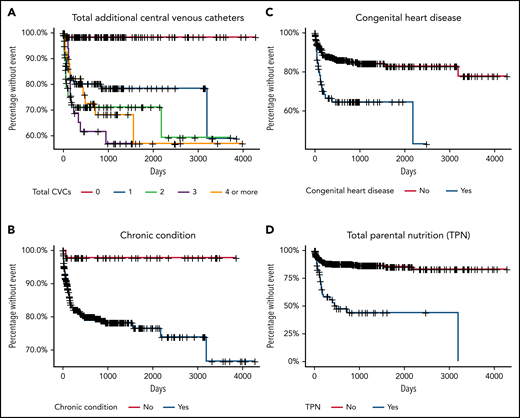
The hazard of developing a recurrent CVC-VTE was modeled using Cox proportional hazards regression (Table 3). On univariate analysis, the placement of at least 1 additional CVC (HR 15.14; 95% CI 3.54-64.78), congenital heart disease (HR 2.90; 95% CI 1.74-4.83), and TPN dependence (HR 5.09; 95% CI 3.08-8.39) were associated with an increased hazard of VTE recurrence (Table 3). On multivariable analysis, these VTE risk factors remained statistically significant: the placement of at least 1 additional CVC (HR 12.00; 95% CI 2.78-51.91), congenital heart disease (HR 3.70; 95% CI 1.97-6.95), and TPN dependence (HR 4.02; 95% CI 2.23-7.28) (Table 3). Figure 1 includes survival curves for selected risk factors.
Univariate and multivariate analysis of risk factors for time until recurrent VTE (n = 373)
| . | Patients without recurrent VTE (n = 308), % . | Patients with recurrent VTE (n = 65), % . | Univariate analysis HR (95% CI) . | Multivariable analysis HR (95% CI) . |
|---|---|---|---|---|
| Age at study entry (y) | ||||
| <1 | 179 (58) | 39 (60) | 1.77 (0.75, 4.19) | 1.09 (0.45, 2.78) |
| 1-5 | 63 (20) | 15 (23) | 1.70 (0.66, 4.40) | 1.63 (0.61, 4.36) |
| 5-12 | 42 (14) | 6 (9) | Reference | Reference |
| >12 | 24 (8) | 5 (8) | 1.57 (0.48, 5.18) | 1.71 (0.51, 5.78) |
| Sex | ||||
| Male | 171 (56) | 32 (49) | Reference | Excluded |
| Female | 137 (44) | 33 (51) | 1.31 (0.81, 2.13) | |
| Race | ||||
| White | 156 (51) | 26 (40) | Reference | Excluded |
| Black | 72 (23) | 23 (35) | 1.68 (0.96, 2.95) | |
| Asian | 14 (5) | 2 (3) | 0.79 (0.19, 3.34) | |
| Other | 66 (21) | 14 (22) | 1.42 (0.74, 2.72) | |
| Total additional CVCs | ||||
| 0 | 132 (43) | 2 (3) | Reference | Reference |
| 1 | 82 (27) | 20 (31) | 15.14 (3.54, 64.78) | 12.00 (2.78, 51.91) |
| 2 | 45 (15) | 18 (28) | 23.74 (5.50, 102.4) | 15.20 (3.43, 67.48) |
| 3 | 21 (7) | 13 (20) | 28.15 (6.34, 125.0) | 14.07 (3.11, 63.58) |
| 4 or more | 28 (9) | 12 (18) | 20.63 (4.62, 92.21) | 11.21 (2.45, 51.41) |
| Chronic medical condition | ||||
| Congenital heart disease | 45 (15) | 23 (35) | 2.90 (1.74, 4.83) | 3.70 (1.97, 6.95) |
| Total parenteral nutrition dependence | 28 (9) | 26 (40) | 5.09 (3.08, 8.39) | 4.02 (2.23, 7.28) |
| Cancer | 29 (9) | 1 (2) | 0.17 (0.02, 1.20) | 0.23 (0.03, 1.81) |
| Renal | 18 (6) | 5 (8) | 1.38 (0.55, 3.43) | Others/reference |
| Hyperinsulinemia | 22 (7) | 2 (3) | 0.69 (0.17, 2.83) | |
| Cystic fibrosis | 5 (2) | 1 (2) | 0.84 (0.12, 6.05) | |
| Autoimmune disease | 2 (1) | 2 (3) | 3.96 (0.97, 16.24) | |
| Gastrointestinal autoimmune disease | 8 (3) | 2 (3) | 0.89 (0.22, 3.68) | |
| Sickle cell disease | 7 (2) | 1 (2) | 0.56 (0.08, 4.05) | |
| None | 158 (51) | 40 (62) | 0.09 (0.01, 0.65) | 0.30 (0.04, 2.30) |
| Congenital thrombophilia* | ||||
| Present | 27 (9) | 9 (14) | 2.10 (0.91, 4.87) | 2.52 (1.05, 6.03) |
| Absent | 161 (52) | 42 (65) | 1.68 (0.92, 3.09) | 1.66 (0.89, 3.13) |
| Not assessed | 120 (39) | 14 (22) | Reference | Reference |
| Acute prothrombotic condition | ||||
| Recent surgery (<30 d) | 60 (19) | 18 (28) | 1.50 (0.87, 2.57) | Excluded |
| Active infection | 63 (20) | 8 (12) | 0.59 (0.28, 1.24) | |
| Protein-losing process | 9 (3) | 2 (3) | 1.17 (0.29, 4.77) | |
| Prothrombotic medication | 11 (4) | 0 (0) | 0† |
| . | Patients without recurrent VTE (n = 308), % . | Patients with recurrent VTE (n = 65), % . | Univariate analysis HR (95% CI) . | Multivariable analysis HR (95% CI) . |
|---|---|---|---|---|
| Age at study entry (y) | ||||
| <1 | 179 (58) | 39 (60) | 1.77 (0.75, 4.19) | 1.09 (0.45, 2.78) |
| 1-5 | 63 (20) | 15 (23) | 1.70 (0.66, 4.40) | 1.63 (0.61, 4.36) |
| 5-12 | 42 (14) | 6 (9) | Reference | Reference |
| >12 | 24 (8) | 5 (8) | 1.57 (0.48, 5.18) | 1.71 (0.51, 5.78) |
| Sex | ||||
| Male | 171 (56) | 32 (49) | Reference | Excluded |
| Female | 137 (44) | 33 (51) | 1.31 (0.81, 2.13) | |
| Race | ||||
| White | 156 (51) | 26 (40) | Reference | Excluded |
| Black | 72 (23) | 23 (35) | 1.68 (0.96, 2.95) | |
| Asian | 14 (5) | 2 (3) | 0.79 (0.19, 3.34) | |
| Other | 66 (21) | 14 (22) | 1.42 (0.74, 2.72) | |
| Total additional CVCs | ||||
| 0 | 132 (43) | 2 (3) | Reference | Reference |
| 1 | 82 (27) | 20 (31) | 15.14 (3.54, 64.78) | 12.00 (2.78, 51.91) |
| 2 | 45 (15) | 18 (28) | 23.74 (5.50, 102.4) | 15.20 (3.43, 67.48) |
| 3 | 21 (7) | 13 (20) | 28.15 (6.34, 125.0) | 14.07 (3.11, 63.58) |
| 4 or more | 28 (9) | 12 (18) | 20.63 (4.62, 92.21) | 11.21 (2.45, 51.41) |
| Chronic medical condition | ||||
| Congenital heart disease | 45 (15) | 23 (35) | 2.90 (1.74, 4.83) | 3.70 (1.97, 6.95) |
| Total parenteral nutrition dependence | 28 (9) | 26 (40) | 5.09 (3.08, 8.39) | 4.02 (2.23, 7.28) |
| Cancer | 29 (9) | 1 (2) | 0.17 (0.02, 1.20) | 0.23 (0.03, 1.81) |
| Renal | 18 (6) | 5 (8) | 1.38 (0.55, 3.43) | Others/reference |
| Hyperinsulinemia | 22 (7) | 2 (3) | 0.69 (0.17, 2.83) | |
| Cystic fibrosis | 5 (2) | 1 (2) | 0.84 (0.12, 6.05) | |
| Autoimmune disease | 2 (1) | 2 (3) | 3.96 (0.97, 16.24) | |
| Gastrointestinal autoimmune disease | 8 (3) | 2 (3) | 0.89 (0.22, 3.68) | |
| Sickle cell disease | 7 (2) | 1 (2) | 0.56 (0.08, 4.05) | |
| None | 158 (51) | 40 (62) | 0.09 (0.01, 0.65) | 0.30 (0.04, 2.30) |
| Congenital thrombophilia* | ||||
| Present | 27 (9) | 9 (14) | 2.10 (0.91, 4.87) | 2.52 (1.05, 6.03) |
| Absent | 161 (52) | 42 (65) | 1.68 (0.92, 3.09) | 1.66 (0.89, 3.13) |
| Not assessed | 120 (39) | 14 (22) | Reference | Reference |
| Acute prothrombotic condition | ||||
| Recent surgery (<30 d) | 60 (19) | 18 (28) | 1.50 (0.87, 2.57) | Excluded |
| Active infection | 63 (20) | 8 (12) | 0.59 (0.28, 1.24) | |
| Protein-losing process | 9 (3) | 2 (3) | 1.17 (0.29, 4.77) | |
| Prothrombotic medication | 11 (4) | 0 (0) | 0† |
Time (d) from initial catheter-associated thrombotic event to additional thrombotic event, or end of follow-up for censored patients.
HR, hazards ratio; VTE, venous thromboembolism.
Congenital thrombophilia defined as factor V Leiden mutation, antithrombin deficiency, protein C deficiency, protein S deficiency, and/or prothrombin gene mutation.
Zero recurrent thrombotic events for patients with prothrombotic medication so HR could not be estimated.
Kaplan-Meier survival curves of days until recurrent thrombosis. Time to VTE recurrence of patients (n = 373) stratified by the number of additional CVCs (A), a chronic condition (as defined in Table 1) (B), congenital heart disease (C), total parenteral nutrition dependence (D).
Kaplan-Meier survival curves of days until recurrent thrombosis. Time to VTE recurrence of patients (n = 373) stratified by the number of additional CVCs (A), a chronic condition (as defined in Table 1) (B), congenital heart disease (C), total parenteral nutrition dependence (D).
The odds of developing a VTE based on additional CVC placement were modeled, with results in Table 4. The non-CVC associated recurrent events were not included in this analysis. On both univariate and multivariate analysis, full-dose anticoagulation was associated with decreased odds of VTE recurrence (OR 0.40; 95% CI 0.23-0.71 and OR 0.35; 95% CI 0.19-0.65). Prophylactic dose anticoagulation was not statistically significant in decreasing the odds of VTE recurrence on either univariate or multivariate analysis (OR 0.60; 95% CI 0.28-1.26 and OR 0.61; 95% CI 0.28-1.30).
Univariate and multivariable analysis of risk factors for CVC-related recurrent VTE or additional catheter placements
| . | CVCs without recurrent VTE (n = 492), % . | CVCs with recurrent VTE (n = 58), % . | Univariate analysis OR (95% CI) . | Multivariable analysis OR (95% CI) . |
|---|---|---|---|---|
| Age at CVC placement | ||||
| <1 | 237 (48) | 32 (55) | 1.83 (0.66, 5.06) | 1.11 (0.37, 3.33) |
| 1-5 | 137 (28) | 18 (31) | 1.77 (0.61, 5.12) | 1.23 (0.38, 3.95) |
| 5-12 | 67 (14) | 5 (9) | Reference | Reference |
| >12 | 51 (10) | 3 (5) | 0.79 (0.18, 3.55) | 0.67 (0.14, 3.24) |
| Sex | ||||
| Male | 260 (53) | 29 (50) | Reference | Excluded |
| Female | 232 (47) | 29 (50) | 1.12 (0.65, 1.94) | |
| Race | ||||
| White | 262 (53) | 22 (38) | Reference | Excluded |
| Black | 100 (20) | 22 (38) | 2.66 (1.42, 4.98) | |
| Asian | 16 (3) | 2 (3) | 1.50 (0.31, 7.29) | |
| Other | 114 (23) | 12 (21) | 1.28 (0.63, 2.61) | |
| CVC type | ||||
| Peripherally inserted central catheter | 310 (63) | 44 (76) | 1.45 (0.69, 3.02) | 1.53 (0.70, 3.34) |
| Tunneled catheter | 80 (16) | 4 (7) | 0.51 (0.15, 1.71) | 0.63 (0.17, 2.38) |
| Nontunneled catheter | 102 (21) | 10 (17) | Reference | Reference |
| Additional CVC placed | ||||
| 1 | 214 (44) | 25 (43) | 1.04 (0.46, 2.37) | Excluded |
| 2 | 124 (25) | 13 (22) | 0.93 (0.37, 2.33) | |
| 3 | 64 (13) | 10 (17) | 1.39 (0.52, 3.71) | |
| 4 or higher | 90 (18) | 10 (17) | Reference | |
| Chronic medical condition | ||||
| Congenital heart disease | 103 (21) | 20 (34) | 1.99 (1.12, 3.53) | 3.02 (1.59, 5.73) |
| Total parenteral nutrition dependence | 100 (20) | 24 (41) | 2.78 (1.62, 4.78) | 3.08 (1.65, 5.74) |
| Cancer | 48 (10) | 1 (2) | 0.16 (0.02, 1.21) | 0.59 (0.06, 5.39) |
| Renal | 51 (10) | 5 (9) | 0.82 (0.30, 2.20) | Others/reference |
| Hyperinsulinemia | 23 (5) | 2 (3) | 0.72 (0.23, 2.32) | |
| Cystic fibrosis | 11 (2) | 1 (2) | 0.77 (0.09, 6.72) | |
| Autoimmune disease | 19 (4) | 1 (2) | 0.40 (0.03, 4.79) | |
| Gastrointestinal autoimmune disease | 34 (7) | 2 (3) | 0.45 (0.08, 2.38) | |
| Sickle cell disease | 3 (1) | 1 (2) | 2.86 (0.24, 33.48) | |
| None | 29 (6) | 1 (2) | 0.28 (0.03, 2.34) | |
| Congenital thrombophilia* | ||||
| Present | 51 (10) | 8 (14) | 1.69 (0.70, 4.12) | 1.81 (0.74, 4.40) |
| Absent | 301 (61) | 37 (64) | 1.33 (0.69, 2.57) | 1.63 (0.81, 3.26) |
| Not assessed | 140 (28) | 13 (22) | Reference | Reference |
| Acute clinical issue | ||||
| Recent surgery (<30 d) | 104 (21) | 17 (29) | 1.55 (0.84, 2.87) | Excluded |
| Active infection | 53 (11) | 5 (9) | 0.67 (0.28, 1.59) | |
| Protein-losing process | 33 (7) | 2 (3) | 0.50 (0.11, 2.23) | |
| Prothrombotic medication | 21 (4) | 0 (0) | 0† | |
| Secondary prophylaxis | ||||
| Prophylactic dosing | 84 (17) | 9 (16) | 0.60 (0.28, 1.26) | 0.61 (0.28, 1.30) |
| Full dosing | 282 (57) | 22 (38) | 0.40 (0.23, 0.71) | 0.35 (0.19, 0.65) |
| No anticoagulation | 141 (29) | 27 (47) | Reference | Reference |
| . | CVCs without recurrent VTE (n = 492), % . | CVCs with recurrent VTE (n = 58), % . | Univariate analysis OR (95% CI) . | Multivariable analysis OR (95% CI) . |
|---|---|---|---|---|
| Age at CVC placement | ||||
| <1 | 237 (48) | 32 (55) | 1.83 (0.66, 5.06) | 1.11 (0.37, 3.33) |
| 1-5 | 137 (28) | 18 (31) | 1.77 (0.61, 5.12) | 1.23 (0.38, 3.95) |
| 5-12 | 67 (14) | 5 (9) | Reference | Reference |
| >12 | 51 (10) | 3 (5) | 0.79 (0.18, 3.55) | 0.67 (0.14, 3.24) |
| Sex | ||||
| Male | 260 (53) | 29 (50) | Reference | Excluded |
| Female | 232 (47) | 29 (50) | 1.12 (0.65, 1.94) | |
| Race | ||||
| White | 262 (53) | 22 (38) | Reference | Excluded |
| Black | 100 (20) | 22 (38) | 2.66 (1.42, 4.98) | |
| Asian | 16 (3) | 2 (3) | 1.50 (0.31, 7.29) | |
| Other | 114 (23) | 12 (21) | 1.28 (0.63, 2.61) | |
| CVC type | ||||
| Peripherally inserted central catheter | 310 (63) | 44 (76) | 1.45 (0.69, 3.02) | 1.53 (0.70, 3.34) |
| Tunneled catheter | 80 (16) | 4 (7) | 0.51 (0.15, 1.71) | 0.63 (0.17, 2.38) |
| Nontunneled catheter | 102 (21) | 10 (17) | Reference | Reference |
| Additional CVC placed | ||||
| 1 | 214 (44) | 25 (43) | 1.04 (0.46, 2.37) | Excluded |
| 2 | 124 (25) | 13 (22) | 0.93 (0.37, 2.33) | |
| 3 | 64 (13) | 10 (17) | 1.39 (0.52, 3.71) | |
| 4 or higher | 90 (18) | 10 (17) | Reference | |
| Chronic medical condition | ||||
| Congenital heart disease | 103 (21) | 20 (34) | 1.99 (1.12, 3.53) | 3.02 (1.59, 5.73) |
| Total parenteral nutrition dependence | 100 (20) | 24 (41) | 2.78 (1.62, 4.78) | 3.08 (1.65, 5.74) |
| Cancer | 48 (10) | 1 (2) | 0.16 (0.02, 1.21) | 0.59 (0.06, 5.39) |
| Renal | 51 (10) | 5 (9) | 0.82 (0.30, 2.20) | Others/reference |
| Hyperinsulinemia | 23 (5) | 2 (3) | 0.72 (0.23, 2.32) | |
| Cystic fibrosis | 11 (2) | 1 (2) | 0.77 (0.09, 6.72) | |
| Autoimmune disease | 19 (4) | 1 (2) | 0.40 (0.03, 4.79) | |
| Gastrointestinal autoimmune disease | 34 (7) | 2 (3) | 0.45 (0.08, 2.38) | |
| Sickle cell disease | 3 (1) | 1 (2) | 2.86 (0.24, 33.48) | |
| None | 29 (6) | 1 (2) | 0.28 (0.03, 2.34) | |
| Congenital thrombophilia* | ||||
| Present | 51 (10) | 8 (14) | 1.69 (0.70, 4.12) | 1.81 (0.74, 4.40) |
| Absent | 301 (61) | 37 (64) | 1.33 (0.69, 2.57) | 1.63 (0.81, 3.26) |
| Not assessed | 140 (28) | 13 (22) | Reference | Reference |
| Acute clinical issue | ||||
| Recent surgery (<30 d) | 104 (21) | 17 (29) | 1.55 (0.84, 2.87) | Excluded |
| Active infection | 53 (11) | 5 (9) | 0.67 (0.28, 1.59) | |
| Protein-losing process | 33 (7) | 2 (3) | 0.50 (0.11, 2.23) | |
| Prothrombotic medication | 21 (4) | 0 (0) | 0† | |
| Secondary prophylaxis | ||||
| Prophylactic dosing | 84 (17) | 9 (16) | 0.60 (0.28, 1.26) | 0.61 (0.28, 1.30) |
| Full dosing | 282 (57) | 22 (38) | 0.40 (0.23, 0.71) | 0.35 (0.19, 0.65) |
| No anticoagulation | 141 (29) | 27 (47) | Reference | Reference |
OR, odds ratio; VTE, venous thromboembolism.
Congenital thrombophilia defined as factor V Leiden mutation, antithrombin deficiency, protein C deficiency, protein S deficiency, and/or prothrombin gene mutation.
Zero events on prothrombotic medication so OR could not be estimated.
Bleeding events during the secondary prophylaxis time period were collected in order to evaluate the harm associated with secondary prophylaxis. Bleeding during the treatment phase was not evaluated. Within the prespecified secondary prophylaxis time period, a total of 153 subsequent CVCs were placed in 78 patients who also received anticoagulation for secondary prophylaxis. There were 2 major and 6 CRNM bleeding events in 8 patients with 8 CVCs. At the time of the 2 major bleeding events, both patients were on a full dose of enoxaparin. One patient was a critically ill, coagulopathic 13-month-old with renal disease who had intracranial bleeding; the other patient was a 20-month-old with congenital heart disease with retroperitoneal bleeding and a fall in hemoglobin >2 g/dL. Neither of the major bleeding events were fatal. At the time of the 6 CRNM bleeding events, 2 patients were on a full dose of enoxaparin, 1 was on a full dose of enoxaparin and aspirin, and 3 were on a prophylactic dose of enoxaparin. There was no statistically significant association between anticoagulation dose and bleeding (Fisher’s exact test, P = .1139). Seventy-five percent (6/8) of major and CRNM bleeding patients were hospitalized at the time of their bleeding event, 50% (4/8) were critically ill. The patient receiving aspirin and a full dose of enoxaparin had a supratherapeutic anti-Xa at the time of bleeding (anti-Xa: 1.13 IU/mL) but had subtherapeutic or therapeutic anti-Xa values drawn in the days prior to the CRNM bleeding event. All other patients with bleeding events had therapeutic anti-Xa levels (0.5-1 IU/mL) or were receiving prophylactic doses with unmonitored or subtherapeutic anti-Xa levels at the time of bleeding or in the 2 weeks prior to the bleeding event. In summary, major bleeding occurred in 1.3% (2/153) of CVCs with anticoagulation for secondary prophylaxis or 3% (2/78) of patients, and CRNM bleeding occurred in 3.9% (6/153) of CVCs with anticoagulation for prophylaxis or 8% (6/78) of patients during the secondary prophylaxis time period while on anticoagulation.
Discussion
Children cared for in tertiary and quaternary care hospitals often have complex chronic medical conditions and/or may be critically ill, requiring the use of CVCs for nutrition, supportive care, chemotherapy, or antibiotics. In our hospital, children who develop CVC-VTE are at high risk for recurrent VTE, particularly with additional CVC placement. The placement of an additional CVC was associated with the highest hazard of VTE recurrence (HR 12.00; 95% CI 2.78-51.91). It is striking that nearly two-thirds of patients in our cohort (64.1%) with an index CVC-VTE required at least 1 additional CVC, highlighting the urgent need for evidence-based clinical guidelines in this scenario.
Debate remains regarding the efficacy of anticoagulation to prevent CVC-VTE. Several studies using prophylactic dosing strategies for primary prophylaxis with CVC placement have not demonstrated efficacy.11-13 A recent phase 2b clinical trial provided new evidence regarding efficacy of prophylactic enoxaparin in critically ill children >1 year of age when initiated within 24 hours of CVC placement, and these findings will be validated in a phase 3 study.14,15 In our study, full-dose anticoagulation as secondary prophylaxis with subsequent CVC placement was associated with a 65% reduction in the odds of CVC-VTE recurrence as compared with no anticoagulation. Prophylactic dosing did not seem to confer a benefit. The largest concern with full-dose anticoagulation as prophylaxis is the associated bleeding risk. We were able to demonstrate low rates of major bleeding in the secondary prophylaxis window (1.3% of CVCs) with no fatal bleeding. Both major bleeding events occurred in hospitalized, critically ill patients with additional risk factors for hemorrhage.
Prior studies have demonstrated PICCs are associated with a higher incidence of VTE compared with tunneled lines.16 Although a majority of our cohort had a PICC-related VTE, PICCs were not associated with recurrent thrombosis on univariate or multivariate analysis compared with nontunneled catheters. There was a trend toward a modest increase in odds of VTE recurrence with the number of additional CVCs placed beyond the first, although the odds are very high, so estimates have a large amount of variability.
Of all the chronic medical conditions analyzed, children with TPN dependence and CHD had the highest hazard for recurrent VTE (HR 4.02; 95% CI 2.23-7.28 and HR 3.70; 95% CI 1.97-6.95, respectively) on multivariate analysis. The 2012 Chest Guidelines recommend thromboprophylaxis for children with TPN dependence using vitamin K antagonists (goal INR 2-3) based on low-quality evidence from small studies.7 Our study results would also support full dose anticoagulation to prevent recurrent VTE in patients with a prior CVC-VTE and TPN dependence.
Notably, 10% of patients in our cohort with an index CVC-VTE and 14% of patients with recurrent VTE were diagnosed with a congenital thrombophilia. However, 36% of patients in the study were not assessed for a thrombophilia. While there is no consensus guideline about thrombophilia testing in pediatrics, it is generally not recommended for a first CVC-VTE and is not part of our routine clinical practice.17,18 Given that over one-third of our cohort did not have thrombophilia testing, we cannot draw conclusions about this population from these results.
Interestingly, 9.2% (6/65) of the recurrent thrombotic events were not associated with a CVC, highlighting the fact that any prior thrombotic event is a risk factor for thrombosis. A prior study by Jaffrey et al demonstrated that a prior history of VTE significantly increased the risk of CVC-VTE; however, the study did not evaluate the risk of recurrent non-CVC related VTE.16 Our results illustrate that a prior history of CVC-VTE also increases the risk of VTE recurrence. Factors that may have exacerbated this risk were sepsis, recent surgery, and major bleeding events necessitating halting anticoagulation prior to the clot recurrence. Current guidelines recommend screening hospitalized pediatric patients ≥12 years for non-CVC associated VTE risk.19-22 Only 2 of the 6 patients with non-CVC associated recurrent VTE would have met this age-based screening criteria. These results suggest that physicians should monitor patients with prior CVC-VTE for clot recurrence while they are in an increased prothrombotic state, regardless of patient age.
The median time to CVC replacement in our cohort was only 4 days. Our clinical practice supports the recommendation to continue to use a necessary and functional CVC, despite VTE.8 However, the primary team ultimately makes the decision about CVC removal based on the specific clinical scenario, which is difficult to capture in this retrospective analysis. The rapid reinsertion of another line in a child who still had an active clot may have contributed to the rate of recurrence found in this study. If functioning lines were not removed, then it is possible that the recurrence rate might be less. Future prospective work should capture the rationale for CVC removal and replacement, as this may affect the risk of recurrent CVC-VTE and have implications for secondary prophylaxis.
Our study has several limitations that must be considered. As this was a single-center, retrospective study and management was not standardized, it is subject to selection bias. Identification of bleeding events via chart review is also a challenge, and clinically relevant nonmajor bleeds may have been missed. Furthermore, 12.6% of the patients included in this study left CHOP before the study period ended. These patients may have had recurrent thrombotic events that we did not capture after they left our institution, leading to an underestimation of the risk of recurrence. Aside from bleeding, we did not assess other side effects of anticoagulation or the burden of potential long-term anticoagulation for patients and families. We believe these are important variables to include in future prospective studies. We also hope prospective work will consider any patient factors that predict receiving full- vs prophylactic-dose anticoagulation to limit confounding by indication. Other future directions include evaluating the effect of secondary prophylactic anticoagulation on central line-associated bloodstream infections (CLABSI), as prior work demonstrates an association between CLABSI and CVC-VTE in pediatrics.16
Children who develop CVC-VTE are at high risk of recurrence, particularly those with subsequent CVC placement. Although our data suggest that full-dose anticoagulation is effective as secondary prophylaxis, these findings should be validated in a prospective randomized multicenter study, with careful assessment of bleeding risk.
Acknowledgment
This study was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grant T32 HL007971 (H.W.).
Authorship
Contribution: H.H.C. performed data collection, wrote the first draft of this manuscript, and revised the manuscript based on the authors’ suggestions; C.W. designed the study, provided oversight for data collection, and revised the manuscript; L.B. contributed to manuscript revision and performed the statistical analysis of data; L.R. and H.W. assisted with study design and manuscript revision; and all authors approved of the content.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Char Witmer, Children’s Hospital of Philadelphia, CTRB, Room 11026, Philadelphia, PA 19104; e-mail: witmer@chop.edu.
For original data, please contact witmer@chop.edu.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal