In this issue of Blood, Shadman et al1 argue that autologous hematopoietic stem cell transplantation (ASCT) may be preferable to chimeric antigen receptor (CAR) T cells for a subset of patients with relapsed diffuse large B-cell lymphoma (DLBCL) who continue to demonstrate chemosensitivity after salvage chemotherapy.
Along with germ cell tumors and select myeloid leukemias, lymphoid malignancies have epitomized chemotherapy-induced cure. If conventional doses fail, dose intensification and stem cell rescue further extend the curative potential of this modality. Indeed, ASCT has been the preferred curative option as second-line treatment of relapsed lymphomas.2 Patients relapsing after ASCT had, until recently, been left without effective options. Over the past few years, anti-CD19 CAR T cells have been a breakthrough, curing even those for whom ASCT fails, leading to the notion that they could supplant ASCT as second-line therapy for those with relapsed DLBCL.3-5 However, Shadman et al argue that ASCT may be preferable to CAR T cells for a subset of such patients with chemosensitivity after salvage chemotherapy.
Results from 2 randomized controlled trials (ZUMA7 [axicabtagene ciloleucel] and TRANSFORM [lisocabtagene maraleucel]) that directly compared both modalities for DLBCL suggest that CAR T cells in the second-line setting may offer superior event-free survival (EFS) and response rates6,7 compared with current standard approaches that include ASCT, thus relegating ASCT to later-stage therapy. In practice, however, we will still see patients who have received salvage chemotherapy (not allowed in ZUMA7 and only 1 cycle in TRANSFORM, which is not typical salvage) before consideration of a cell therapy, who do not fit the early chemotherapy failure criteria in those trials (ie, refractory to or relapsed within 12 months of first-line chemotherapy), or who cannot readily access a CAR T-cell center. Indeed, the EFS was not different between the standard of care and CAR T cells in the BELINDA trial, which had a design similar to those of ZUMA-7 and TRANSFORM except that it permitted standard salvage chemotherapy (~48% of patients received 2 or more salvage cycles) prior to administration of another potent CD19 CAR T-cell product, tisagenlecleucel.8 Furthermore, once CAR T cells are approved as second-line treatment in DLBCL, a randomized trial including only chemosensitive patients with DLBCL is unlikely to be conducted, leaving a void to guide practice in this subset of patients.
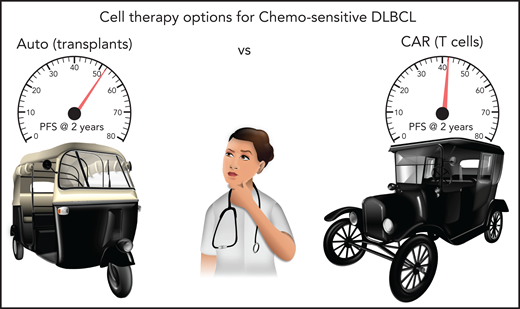
To this end, Shadman et al performed a Center for International Blood and Marrow Transplant Research (CIBMTR)–supported comparative analysis where they demonstrate that those who underwent ASCT while in partial response (PR) after chemotherapy had a lower risk of relapse (40% vs 53% at 2 years) and superior overall survival (69% vs 47% at 2 years) compared with those who received CAR T cells. Of note, progression-free survival, which was the primary end point of the study, was not significantly different (see figure). Therefore, they conclude that ASCT is a suitable option as second-line therapy for DLBCL in PR.
Shadman et al show that progression-free survival (PFS) with ASCT (52% at 2 years) and CAR T cells (42% at 2 years) are comparable (P = .1) for patients with DLBCL in PR after salvage chemotherapy. Multivariable regression and propensity risk matching confirm these trends, thus indicating both cell therapies are viable options for such patients. In this picture, ASCT is represented by an autorickshaw, fondly known as an autos in parts of Southeast Asia, while CAR T cells are represented by a car. Both modes of transportation coexist in many parts of the world.
Shadman et al show that progression-free survival (PFS) with ASCT (52% at 2 years) and CAR T cells (42% at 2 years) are comparable (P = .1) for patients with DLBCL in PR after salvage chemotherapy. Multivariable regression and propensity risk matching confirm these trends, thus indicating both cell therapies are viable options for such patients. In this picture, ASCT is represented by an autorickshaw, fondly known as an autos in parts of Southeast Asia, while CAR T cells are represented by a car. Both modes of transportation coexist in many parts of the world.
The success of ASCT has always depended on the chemosensitivity of tumors, whereas all 3 commercial CAR T-cell products for DLBCL induced unprecedented complete remission rates (30% to 50%) in patients with predominantly chemorefractory DLBCL.3-5 Consistent with our expectations, in this report, ASCT continues to perform splendidly against CAR T cells for chemosensitive DLBCL, thus renewing support for ASCT in the second-line setting.
Results from ZUMA7 and TRANSFORM show a marked difference in median EFS between CAR T cells and standard treatments (8.3 months in ZUMA7 and 10 months in TRANSFORM vs 2 months with standard treatments).6,7 By contrast, the report by Shadman et al indicates that the same therapeutic (axicabtagene ciloleucel) is nearly equivalent to ASCT. There are some obvious explanations for these differing results. First, the ZUMA7 and TRANSFORM prospective trials included only patients at highest risk of chemorefractoriness, whereas Shadman et al included only those who had demonstrated chemosensitivity during salvage treatment. Second, this was a retrospective analysis, with well-established caveats such as matching groups for degree of chemorefractoriness, which were different in this report. Specifically, differing numbers of prior lines of therapy (median, 3 vs 2 for CAR T cells compared with ASCT) and burden of disease at the time of treatment (41% vs 29% with lesions >5 cm for CAR T cells compared with ASCT) could indicate that CAR T-cell recipients were more chemorefractory. Notably, Shadman et al astutely recognized these potential confounders and made every possible effort to match for these characteristics by matching disease burden, matching for the median number of prior lines of therapy, and performing a propensity risk score–matched analyses, and they still demonstrate that ASCT is equivalent to CAR T cells for DLBCL in PR after chemotherapy. Finally, another interpretation of the differences noted here is that the potency of CAR T cells could be enhanced if generated from patients earlier in their treatment course. In ZUMA7 and TRANSFORM, CAR T-cell products were made after just 1 line of therapy, in contrast to ≥2 in this CIBMTR report; thus, the products had fitter starting material. Additional studies will be needed to explore this possibility.
As CAR T cells are now poised to overtake salvage chemotherapy and ASCT in the second-line setting, at least for those with chemorefractory disease, this timely report from Shadman et al argues for maintaining the standard approach for those with chemosensitive DLBCL. Thus, patients and their physicians could have the welcome problem of cell therapy options to choose from. In conclusion, both curative cellular therapies with distinct mechanisms of action are likely to coexist as options for DLBCL. Future research will focus on refinement of their indications.
Conflict-of-interest disclosure: P.D.L. reports advisory board participation for Karyopharm.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal