Peripheral T-cell non-Hodgkin lymphoma (PTCL) represents a heterogeneous group of lymphomas with historically poor prognosis for most subtypes, with the exception of ALK+ anaplastic large-cell lymphoma (ALCL).1 Although the chemotherapy used for B-cell lymphomas, such as cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP), has been the standard of care for many years, the long-term disease-free survival (DFS) and overall survival (OS) are poor.2 One strategy used to improve these outcomes has been the addition of etoposide to CHOP (CHOEP), which has improved the DFS in some subtypes of PTCL in some smaller studies3; and another strategy includes the use of high-dose chemotherapy and autologous peripheral stem cell transplantation (ASCT) in the first complete remission (CR1).4 A large randomized trial in a homogenous patient population to confirm these modifications has not been undertaken. In this issue of Blood, Brink et al5 analyze data from a nationwide population-based Netherlands Cancer Registry to evaluate this point.
In an extensive analysis, Brink at al evaluated patients 18 to 65 years of age with stage II to IV ALCL, angioimmunoblastic T-cell lymphoma (AITL), or PTCL and grouped the patients into 5 broad categories: (1) chemotherapy followed by consolidation with ASCT, (2) chemotherapy without transplantation, (3) a combination of chemotherapy and radiotherapy, and (4) no antineoplastic treatment. A total of 1427 patients were included in their extensive analysis divided into 2 study cohorts: those treated from 1989 through 2008 and those treated from 2009 through 2018.
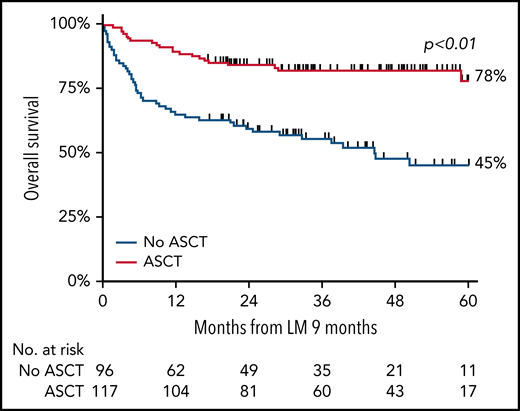
Various analyses were performed in the patient subpopulations. The analysis of the impact of the addition of etoposide to CHOP (CHOEP) revealed an improvement in OS compared with that obtained with CHOP. However, when adjusted for age, PTCL subtype, International Prognostic Index Score, and no stem cell transplantation, the risk of mortality was similar. In a landmark analysis, the 5-year OS of patients who underwent consolidation with ASCT was 78% vs 45% without ASCT (P < .01; see figure). A superior 5-year OS with ASCT consolidation was also observed for each subtype separately, with the exception of ALK+ALCL.
Overall survival of patients <65 years of age with stage II to IV ALCL, AITL, or PTCL treated with or without consolidation with ASCT after first-line chemotherapy. See Figure 4A in the article by Brink et al that begins on page 1009.
Overall survival of patients <65 years of age with stage II to IV ALCL, AITL, or PTCL treated with or without consolidation with ASCT after first-line chemotherapy. See Figure 4A in the article by Brink et al that begins on page 1009.
In the past few years, brentuximab vedotin (BV) when combined with CHP chemotherapy (BV+ cyclophosphamide, doxorubicin, and prednisone) has been the only treatment demonstrated to improve the outcome of patients with newly diagnosed CD30+ PTCL. However, in a retrospective analysis of patients with PTCL treated with BV-CHP, ASCT performed in CR1 still improved OS.6,7 Unfortunately, for patients with PTCL who are not CD30+, no new therapies have been found that improve their survival.
Population-based cancer registry data are not a prospective randomized trial; however, this analysis contains data from a large number of patients, and the results are very convincing. Although the reasons for not taking patients to transplant in CR1 are not available in the registry, it does present real-world data that heavily favor ASCT in CR1 for eligible patients. The strong data continue to support the use of ASCT in CR1 for the common types of PTCL: PTCL-not otherwise specified, AITL, and ALK− ALCL subtypes. The use of ASCT in CR1 should also be considered in patients with PTCL who are >65 years of age, as some patients in this age group would also be transplant eligible and may benefit from this treatment. Unfortunately, many patients with PTCL do not achieve CR1 with any induction chemotherapy, and the benefit of ASCT for them is less than for those in CR1, as demonstrated in this population-based study.
We can hope that someday science-driven analysis and well-designed clinical trials will find targeted agents to enhance the outcomes of patients with PTCL. In the meantime, based on the available data from this registry, ASCT in CR1 should be considered for all eligible patients with PTCL.
Conflict-of-interest: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal