Abstract
Background: Clinical trial design and regulatory approvals of multiple myeloma (MM) drugs with new mechanisms of action often define populations of patients with RRMM based on number of prior LOTs. The premise of such approach is the number of prior LOTs properly identifies patients with uniformly unfavorable prognosis with existing treatments. However, in an environment of fast drug development and assimilation of new agents earlier in therapy, such promise may not be correct. We hypothesized that drug class refractoriness, rather than number of prior LOTs, better classify patients with RRMM undergoing modern therapy.
Methods: We retrospectively analyzed patients starting new, non-experimental regimen for RRMM between 2016 and 2022. For each regimen initiated we extracted patient and disease characteristics, time from MM diagnosis until initiation of the regimen, number of prior LOTs and refractoriness to each 3 main classes of anti-myeloma agents: immunomodulatory drugs (IMiDs) proteasome inhibitors (PIs), and anti-CD38 monoclonal antibodies (anti-CD38 MoABs). We grouped prior LOTs in 1, 2/3 or 4+ and refractoriness in no/single-class refractory (N/SCR), double-class refractory (DCR) and triple-class refractory (TCR). For each regimen we captured treatment response by International Myeloma Working Group (IMWG) criteria and progression-free survival (PFS). We evaluated the impact of prior LOT and drug refractoriness on overall response rate (ORR) and PFS by univariable and multivarible analysis.
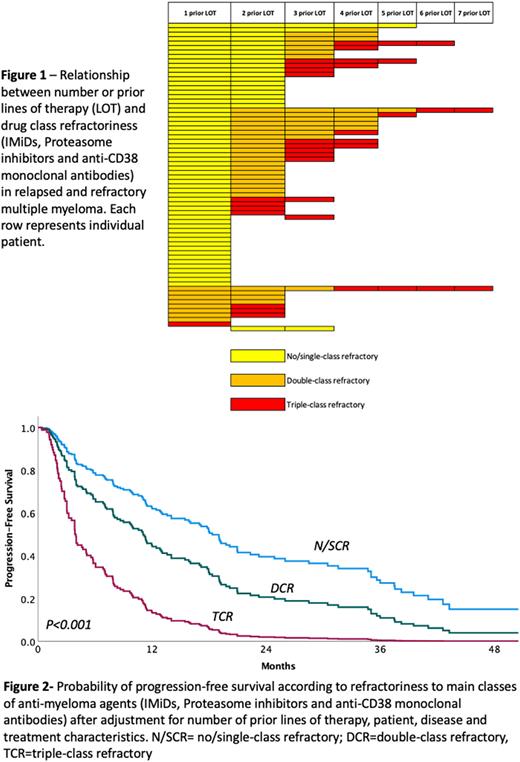
Results: We included 69 patients who received 183 distinct regimens for RRMM. Median age at onset of regimen of interest was 57 years, 46% of patients were of racial/ethnic minorities and 71% had received prior autologous stem cell transplantation. Among patients initiating a new regimen after 1 prior LOT, 88% had N/SCR, 11% DCR and 1% TCR MM. Among patients treated after 2/3 prior LOT 28%, 47% and 25% had N/SCR, DCR and TCR MM respectively. For patients treated after 4+ prior LOT 7%, 30% and 63% had N/SCR, DCR and TCR MM respectively (Figure 1). Median time between MM diagnosis and initiation of the regimen of interest was 39.0 mo. for patients with N/SCR, 45.9 mo. for DCR and 52.1 mo. for TCR MM. An anti-CD38 MoAb was part 51% of regimens employed for N/SCR, 53% for DCR and 35% for TCR MM. Carfilzomib was utilized in 32%, 31% and 45%; Pomalidomide in 32%, 40%, and 36%; and alkylating agents in 22%, 20% and 32% of regimens for N/SCR, DCR and TCR MM respectively. ORR was 65%, 56% and 36% for patients with 1, 2/3 or 4+ prior LOT, and 74%, 53% and 23% for N/SCR, DCR and TCR MM, respectively. In multivariable analysis including patient, disease and treatment characteristics, the number of prior LOT was not associated with ORR (P=0.11). Drug-class refractoriness was strongly associated with ORR (P<0.001), when compared to regimens administered for N/SCR MM, OR for DCR was 0.16 (95% C.I. 0.06-0.48) and OR for TCR was 0.04 (95% C.I. 0.01-0.16). Median PFS was 16.0 mo. for regimens administered after 1 LOT, 11.0 mo. for regimens administered after 2/3 LOT and 6.1 mo. for regimens administered after 4+ LOT. In terms of drug class refractoriness, median PFS was 17.8 mo. for regimens administered for patients with N/SCR, 7.2 mo. for DCR and 3.0 mo. for TCR MM. In multivariable analysis number of prior LOT was not associated with PFS (P=0.16). Drug-class refractoriness strongly predicted PFS (P<0.001). Using regimens administered for N/SCR MM as reference, HR for DCR MM regimens was 1.93 (95% C.I. 1.12-3.33) and HR for TCR MM regimens was 4.83 (95% C.I. 2.50-9.31)- Figure 2.
Conclusion: Among patients receiving modern therapy for RRMM, drug class-refractoriness, but not number of prior LOT predicts ORR and PFS. Clinical trial design and regulatory approvals should identify unmet medical need based on drug-class refractoriness and not consider number of prior LOT. Current approvals based on LOT are likely to leave a large proportion of patients without adequate treatment options, yet without the minimal qualifying number of prior therapies, compromising treatment access.
Disclosures
Costa:Genentech: Research Funding; AbbVie: Research Funding; Sanofi: Consultancy, Honoraria; Bristol Myers Squibb: Consultancy, Honoraria, Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding. Giri:CareVive: Honoraria, Research Funding; OncLive: Honoraria; Pack Health: Research Funding. Bal:Adaptive Biotechnologies: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal