Abstract
Background: Systemic AL amyloidosis is caused by the deposition of misfolded monoclonal immunoglobulin serum free light chains (sFLC) in various organs. Treatment of AL amyloidosis relies mainly on chemotherapy aimed at suppressing the underlying secreting plasma cell clone. Organ responses and survival are greatly influenced by the degree of hematological response evaluated by the decrease in sFLC. Over the last 2 years, anti CD38 monoclonal antibodies (mAb), such as daratumumab (DARA), have emerged as breakthrough targeted therapies for patients (pts) with AL amyloidosis and DARA received approval in combination with CyBorD in frontline pts. Unfortunately, some pts will still not achieve complete response (CR) or ultimately relapse. BCL-2 inhibition represents a promising therapeutic approach in plasma cells dyscrasia and venetoclax (VEN) is an oral BCL-2-selective BH3 mimetic. VEN has shown activity in multiple myeloma (MM) cell lines with t(11;14), partly because of greater BCL-2 co-dependence of such clones. VEN is safe in MM pts with encouraging clinical efficacy. VEN should provide a novel targeted therapy in AL amyloidosis as 50-60% of the pts have a t(11;14). Various retrospective cohorts are now reported with VEN alone or in combination with bortezomib (BOR) and/or DARA, with rapid and deep hematological and organ responses.
Objective: To report efficacy of VEN based regimen in AL amyloidosis pts as consolidation or relapsed therapy in frontline AL pts treated with DARA-CyBorD or equivalent.
Material & Methods: In this retrospective study, we reviewed charts of 9 pts with AL amyloidosis, from the French Amyloidosis Network, who received VEN as part of their regimen as consolidation (n=3) or for biological progressive disease (n=6) after DARA-CyBorD frontline therapy. Individual data were prospectively and retrospectively collected in the French Amyloidosis database. Pts should have received at least 1 cycle of VEN-containing regimen to be analyzed. Responses were reviewed using current criteria for CR (serum and urine negative immunofixation and normal involved FLC) and for very good partial response (VGPR;differential FLC<40 mg/l).
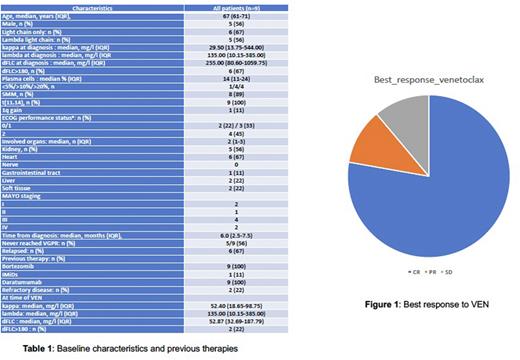
Results: Regarding baseline characteristics (Table 1), pts had a median age of 67 years (IQR:61-71) and all except one had more than 10% bone marrow plasma cells (but no overt MM). Six pts had ≥2 organs involved with heart in 67 % and kidney in 56 %; 6 had MAYO stage 3/4 and median NT-proBNP/troponin levels were 3333 ng/l (IQR:185-9685) and 44 pg/ml (IQR:18-96), respectively. Six pts had FLC only AL amyloidosis. Median dFLC for the whole cohort was 255 mg/l (IQR:140-1060), and 6 pts had baseline dFLC>180 mg/l. All pts had t(11;14) with an additional 11q gain in 3, and 1q gain in 1.
Median time from diagnosis to VEN therapy was 6.0 months (IQR: 2.5-7.5). Regarding frontline therapy, 7 pts received DARA-CyborD (median 3 cycles), 1 DARA-CyBorD plus CAEL and 1 pt received DARA-RVD. All pts were DARA exposed, 8 pts had a suboptimal response to DARA and 2 were DARA "refractory". Five pts never reached VGPR or better with DARA-based regimen.
VEN (400 mg/day) was given alone in 1 pt or in association with dexamethasone/DEXA (n=1), BOR (n=2), or DARA+BOR (n=5); Median time on therapy was 7.0 months (IQR:3.0-9.5). At data cut-off (06/2022), 6 pts had discontinued therapy because of CR (n=5) and/or GI tract toxicity (n=4). The hematologic response rate was 89%; 78% of pts achieved CR, and 11% partial response (PR) (Figure 1). Among CR pts, all pts achieved dFLC<10 mg/l, and 2 had an iFLC<10 mg/l. Responses were fast and median time to CR was 1 month (IQR:1-3).
Regarding VEN monotherapy +/_ DEXA, 50% of pts reached CR; with the addition of BOR, or DARA+BOR, CR was 100, and 80%, respectively. In consolidation pts, all pts achieved CR in 1 month (1-3) with the addition of VEN to DARA-CyBorD. In progressive/relapsed pts, 4/6 (67%) achieved CR and 1 was in stable disease. One pt reached PR and progressed to end-stage renal disease. Finally, both DARA R/R pts reached CR with VEN alone or in association with BOR.
Summary & Conclusion: Venetoclax is a promising targeted therapy in AL amyloidosis. Venetoclax can be used in combination with daratumumab and/or bortezomib as consolidation or relapsed therapy after frontline DARA-CyBorD, even in refractory patients, with expected good safety profile.
Disclosures
Roussel:BMS: Honoraria, Research Funding; SANOFI: Research Funding; janssen: Honoraria, Other: travel fees, Research Funding. Decaux:Gilead: Honoraria; Roche: Honoraria; Takeda: Honoraria; Sanofi: Honoraria; GSK: Honoraria; BMS: Honoraria; Janssen: Honoraria. Bridoux:Janssen: Consultancy, Honoraria, Other: travel fees, Speakers Bureau; SANOFI: Research Funding, Speakers Bureau; ASTRA ZENECA: Consultancy, Speakers Bureau. Jaccard:Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; sanofi: Research Funding; Amgen: Honoraria; Pfizer: Honoraria.
OffLabel Disclosure:
venetoclax in AL amyloidosis
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal