Abstract
Introduction: DARA as monotherapy and combination therapy is approved across lines of treatment for multiple myeloma. The single-arm phase 2 MASTER study (NCT03224507) evaluated DARA + carfilzomib/lenalidomide/dexamethasone (D-KRd), which demonstrated promising clinical efficacy in transplant-eligible NDMM. The primary endpoint analysis (median follow-up, 25.1 mo) showed that minimal residual disease (MRD) negativity at the 10-5 threshold was achieved by 80% of D-KRd pts(Costa, et al. JCO. 2021). The randomized phase 2 GRIFFIN study (NCT02874742) evaluated DARA + lenalidomide/bortezomib/dexamethasone (D-RVd) in transplant-eligible NDMM; the primary endpoint analysis (median follow-up, 13.5 mo) showed that the rate of stringent complete response by the end of consolidation was significantly higher for D-RVd versus RVd (42.4% vs 32.0%; 1-sided P = 0.068, meeting the prespecified 1-sided α of 0.1) (Voorhees, et al. Blood. 2020). Here, we present an analysis of pts with HRCA, defined as having ≥1 genetic abnormality: del17p, t(4;14), t(14;16), t(14;20), and/or gain/amp1q (≥3 copies of chromosome 1q21) from MASTER (median follow-up, 31.1 mo) and GRIFFIN (median follow-up, 49.6 mo).
Methods: In MASTER, enrolled pts (no age limit with enrichment for high-risk disease) received 4 D-KRd induction cycles, autologous stem cell transplant (ASCT), and 0, 4 or 8 D-KRd consolidation cycles with treatment cessation upon achievement of 2 consecutive MRD-negative assessments, or lenalidomide (R) maintenance therapy if 2 consecutive MRD-negative assessments were not achieved. Pts received 28-day cycles of K (20/56 mg/m2 IV Days [D] 1, 8, 15), R (25 mg PO D1-21), d (40 mg PO or IV D1, 8, 15, and 22), and DARA (16 mg/kg IV: D1, 8, 15, and 22 for Cycles 1-2; D1 and 15 for Cycles 3-6; and D1 for Cycles 7+). In GRIFFIN, enrolled pts (age ≤70 years) were randomized 1:1 to receive 4 D-RVd/RVd induction cycles, ASCT, 2 D-RVd/RVd consolidation cycles, and up to 2 years of maintenance with R ± DARA. For induction/consolidation (21-day cycles), pts received R (25 mg PO on Days 1-14), V (1.3 mg/m2 SC on Days 1, 4, 8, and 11), and d (40 mg PO QW) ± DARA (16 mg/kg IV QW in Cycles 1-4 and D1 of Cycles 5-6). During maintenance (28-day cycles), pts received R (10 mg PO D1-21; if tolerated, 15 mg Cycles 10+) ± DARA (16 mg/kg IV Q8W or Q4W, or 1800 mg SC Q4W per protocol amendments). Following completion of study therapy, pts could continue R maintenance per local standard of care. Before the randomized phase, a safety run-in was conducted to assess D-RVd dose-limiting toxicities.
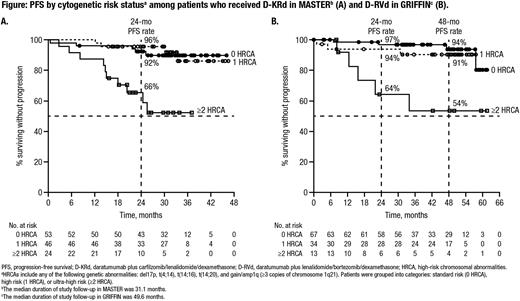
Results: Among pts in MASTER (D-KRd, n = 123), 43% (n = 53) had standard risk (0 HRCA), 37% (n = 46) had high risk (1 HRCA), and 20% (n = 24) had ultra-high risk (≥2 HRCA) NDMM. Among 120 pts in GRIFFIN who received D-RVd (n = 104 randomized phase pts and n = 16 safety run-in pts), 56% (n = 67) had NDMM with 0 HRCA, 28% (n = 34) had 1 HRCA, 11% (n = 13) had ≥2 HRCA, and 5% (n = 6) were not evaluable. In an analysis of best response on study, rates of complete response or better (≥CR) for pts with 0, 1, and ≥2 HRCA were 91%, 89%, and 71% for D-KRd pts in MASTER, respectively, and 91%, 79%, and 62% for D-RVd pts in GRIFFIN. MRD-negativity rates at the 10-5 threshold were 80%, 86%, and 83% for D-KRd for pts with 0, 1, and ≥2 HRCA, respectively, and MRD-negativity rates at the 10-6 threshold were 68%, 80%, and 67%. For D-RVd pts, MRD-negativity rates at the 10-5 threshold were 76%, 56%, and 62% for pts with 0, 1, and ≥2 HRCA, respectively, and MRD-negativity rates at 10-6 were 45%, 26%, and 15%. MRD negativity (10‒5) with ≥CR occurred in 76%, 79%, and 67% of D-KRd pts with 0, 1, and ≥2 HRCA, respectively, and 75%, 53%, and 54% of D-RVd pts. In MASTER, 24-month progression-free survival (PFS) rates were 92%, 96%, and 66% for D-KRd pts with 0, 1, and ≥2 HRCA, respectively (Figure A). In GRIFFIN, 24-month PFS rates were 97%, 94%, and 64% for D-RVd pts with 0, 1, ≥2 HRCA, respectively, and 48-month PFS rates were 94%, 91%, and 54% (Figure B).
Conclusions: In MASTER and GRIFFIN, pts with NDMM and 0 HRCA or 1 HRCA who received DARA-based quadruplet therapy achieved high rates of ≥CR, MRD negativity, and 2-year PFS rates. These data support use of DARA-based quadruplet therapy as frontline treatment among pts with high cytogenetic risk. However, among pts with NDMM and ≥2 HRCA, results were not similar, and the subgroup was small suggesting that more research is needed for pts with the poor prognosis of ultra-high-risk disease.
Disclosures
Silbermann:Sanofi-Aventis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees. Kaufman:AbbVie, Genentech, and Bristol Myers Squibb: Consultancy; AbbVie: Other: Member of steering committee; Incyte: Other: Member of data safety monitoring committee . Laubach:Lignancies: Honoraria. Schmidt:Sanofi: Consultancy; Janssen: Consultancy. Sborov:GlaxoSmithKline, Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Skyline Dx, Janssen, AbbVie, Sanofi: Consultancy; BMS: Consultancy. Reeves:Incyte, BMS, PharmaEssentia, CTI Biopharma: Honoraria; Hemostasis & Thrombosis Research Society Mentored Research Award sponsored by CSL Behring: Research Funding. Dhakal:BMS: Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding, Speakers Bureau; Amgen: Consultancy, Honoraria; GlaxoSmithKline: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria, Research Funding, Speakers Bureau; Karyopharm Therapeutics: Honoraria, Speakers Bureau; Natera: Consultancy; Arcellx: Research Funding; Carsgen: Research Funding; Cartesian: Research Funding; Fate: Research Funding; Takeda: Honoraria; Pfizer: Membership on an entity's Board of Directors or advisory committees; AbbVie: Membership on an entity's Board of Directors or advisory committees; Genentech: Membership on an entity's Board of Directors or advisory committees. Rodriguez:Janssen, BMS, Takeda, AbbVie, karyopharm, Artiva: Consultancy, Speakers Bureau. Chhabra:Sanofi: Research Funding; Amgen: Research Funding; Janssen: Research Funding; GlaxoSmithKline: Honoraria. Anderson:Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; GSK: Honoraria, Membership on an entity's Board of Directors or advisory committees; Prothena: Honoraria, Membership on an entity's Board of Directors or advisory committees; Karyopharm: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Sanofi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Beigene: Honoraria, Membership on an entity's Board of Directors or advisory committees. Dholaria:Vanderbilt University Medical Center: Current Employment; Gamida Cell: Consultancy; BEAM Therapeutics: Consultancy; Wugen: Research Funding; Angiocrine: Research Funding; Orca Bio: Research Funding; Arivan: Consultancy; Molecular Templates: Research Funding; MJH Biosciences: Honoraria; Janssen: Research Funding; Pfizer: Research Funding; Takeda: Research Funding; Poseida: Research Funding; Jazz Pharmaceuticals: Consultancy; BMS: Research Funding; MEI Pharma: Research Funding. Hari:Takeda: Consultancy, Honoraria; Incyte: Honoraria; Kite: Consultancy, Honoraria; GlaxoSmithKline: Honoraria; Novartis: Honoraria; BMS: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria; Karyopharm: Consultancy, Honoraria; Spectrum Pharmaceuticals: Research Funding; Iovance: Current Employment; Sanofi: Consultancy, Honoraria; AbbVie: Honoraria; Pharmacyclics: Consultancy; Millennium: Research Funding; Amgen: Consultancy, Honoraria. Shah:GSK, Amgen, Indapta Therapeutics, Sanofi, CareDx, Kite, Karyopharm, Oncopeptides,: Consultancy; AstraZeneca: Current Employment, Current equity holder in publicly-traded company; Aztra Zeneca: Current Employment, Other: stock ownership; Celgene/BMS, Janssen, Bluebird Bio, Sutro Biopharma, Teneobio, Poseida, Nektar, Precision Biosciences: Research Funding. Bumma:Janssen: Membership on an entity's Board of Directors or advisory committees, Other: Ad Board; Sanofi, Genzyme: Other: Ad Board, Speakers Bureau; Amgen: Consultancy, Speakers Bureau. Holstein:GSK: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; BMS/Celgene: Consultancy, Research Funding; Sanofi: Consultancy; Takeda: Consultancy, Research Funding; Oncopeptides: Consultancy, Research Funding; Secura Bio: Consultancy; Genentech: Consultancy. Costello:BMS, Takeda, Janssen, Pfizer: Honoraria, Research Funding. Jakubowiak:Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Wildes:Janssen: Consultancy; Carevive: Consultancy; Seattle Genetics: Consultancy; Sanofi: Consultancy. Orlowski:Abbvie, BioTheryX, Inc., Bristol-Myers Squibb, Janssen Biotech, Karyopharm Therapeutics, Inc., Meridian Therapeutics, Monte Rosa Therapeutics, Neoleukin Corporation, Oncopeptides AB, Regeneron Pharmaceuticals, Inc., Sanofi-Aventis, and Takeda Pharmaceutic: Honoraria, Membership on an entity's Board of Directors or advisory committees; Asylia Therapeutics, Inc.: Current equity holder in private company; CARsgen Therapeutics, Celgene/Bristol Myers Squibb, Exelixis, Janssen Biotech, Sanofi-Aventis, Takeda Pharmaceuticals North America, Inc.: Research Funding; Asylia Therapeutics, Inc., BioTheryX, Inc., Heidelberg Pharma, Inc.: Research Funding. Shain:Bristol Myers Squibb (BMS), Janssen, GlaxoSmithKline (GSK), Adaptive, Sanofi, and Takeda, and Amgen: Honoraria; GSK, Janssen and BMS: Membership on an entity's Board of Directors or advisory committees; GSK, BMS, Sanofi, Karyopharm, Takeda, Janssen, Adaptive and Amgen: Speakers Bureau; AbbVie and Karyopharm: Research Funding; Janssen and BMS: Other: PI of clinical trials. Cowan:Secura bio: Consultancy; Adaptive Biotechnologies: Membership on an entity's Board of Directors or advisory committees; GSK: Consultancy; EUSA: Consultancy; Allogene: Consultancy; AbbVie: Consultancy, Research Funding; Nektar: Research Funding; BMS: Consultancy, Research Funding; Sanofi-Aventis: Research Funding; Harpoon: Research Funding; Janssen: Consultancy, Research Funding. Pei:Janssen: Current Employment, Current equity holder in publicly-traded company. Cortoos:Janssen: Current Employment, Current equity holder in publicly-traded company. Patel:Companies of Johnson & Johnson: Current Employment, Current equity holder in publicly-traded company. Lin:Janssen: Current Employment, Current holder of stock options in a privately-held company. Giri:OncLive: Honoraria; CareVive: Honoraria, Research Funding; Pack Health: Research Funding. Costa:Bristol Myers Squibb: Consultancy, Honoraria, Research Funding; AbbVie: Research Funding; Genentech: Research Funding; Adaptive Biotechnologies: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Honoraria, Research Funding. Richardson:Oncopeptides: Consultancy, Research Funding; Protocol Intelligence: Consultancy; Karyopharm: Consultancy, Research Funding; Sanofi: Consultancy; Secura Bio: Consultancy; GlaxoSmithKline: Consultancy; Regeneron: Consultancy; AstraZeneca: Consultancy; Takeda, Celgene, and GSK: Honoraria; Takeda: Research Funding; Takeda, Abbvie, GSK, and Celgene: Consultancy; Takeda: Consultancy, Research Funding; Celgene/BMS: Consultancy, Research Funding; Takeda and GSK: Other: Travel expenses from Takeda and GSK. Usmani:Abbvie, Amgen, BMS, Celgene, EdoPharma, Genentech, Gilead, GSK, Janssen,Oncopeptides, Sanofi, Seattle Genetics, SecuraBio, SkylineDX, Takeda, TeneoBio: Consultancy; Amgen, BMS, Janssen, Sanofi: Speakers Bureau; Amgen, Array Biopharma, BMS, Celgene, GSK, Janssen, Merck, Pharmacyclics, Sanofi, Seattle Genetics, SkylineDX, Takeda: Research Funding. Voorhees:Abbvie, Amgen, BMS, GSK, Karyopharm, Novartis, Oncopeptides, Pfizer, Sanofi, SecuraBio: Consultancy, Honoraria.
OffLabel Disclosure:
The specific regimen combination is not yet approved, but individual components are
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal