Abstract
Introduction Mantle cell lymphoma (MCL) accounts for 4-6% of B-cell non-Hodgkin lymphoma (NHL), with historically poor outcomes. With the advent of targeted and cellular therapies, responses and durable remissions have improved although cure remains elusive. As patients experience longer remissions, this raises the question of the optimal surveillance modality. For NHL in general, surveillance imaging surveillance does not improve outcomes and is associated with increased radiation exposure, high costs, and anxiety to patients.
Peripheral blood minimal residual disease (MRD) analysis offers a potential alternative to surveillance imaging that is more sensitive, less costly, and eliminates the risk of radiation exposure. The clonoSEQ® assay (Adaptive Biotechnologies) is an FDA-cleared Ig-HTS MRD assay which takes advantage of the fact that B-cell lymphomas nearly always have one or more unique clonal immunoglobulin (Ig) gene rearrangements which can serve as a tumor "marker". These sequences can be detected in the peripheral blood, as circulating tumor DNA (ctDNA), with a sensitivity as low as 1 cell in 1,000,000.
Starting in 2015, our group has monitored many of our MCL patients using the clonoSEQ® MRD assay. This has been done in lieu of surveillance imaging, or to minimize its use. We performed a retrospective analysis to summarize our experience with this assay to demonstrate its utility as a surveillance tool for MCL patients.
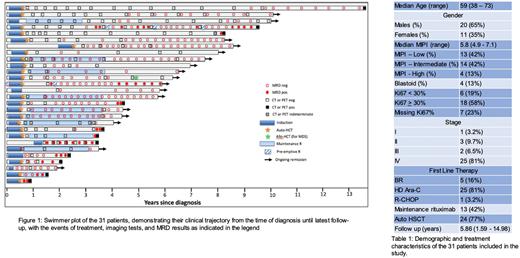
Methods We conducted retrospective data collection and analysis of outcomes in patients who underwent first-line treatment for MCL and were then monitored post-treatment using the clonoSEQ® assay. We captured baseline patient characteristics, as well as every time a surveillance imaging or ctDNA (clonoSEQ®) test was performed. We used swimmer plots to display timelines for individual patients, indicating all ctDNA tests (positive or negative), CT or PET scan results, as well as documented clinical relapse. We used Kaplan-Meier curves to show progression-free survival and overall survival of the entire cohort. We compared ctDNA to radiographic surveillance in terms of ability to detect relapse.
Results A total of 31 patients were included. The median age of patients was 59, and the majority had low- or intermediate-risk disease by MIPI score, with 58% having a proliferation index above 30%. The majority (81%) underwent high-dose cytarabine-based induction, 77% underwent autologous transplant in first remission, and 42% received rituximab maintenance (see Table 1). With a median follow up of 5.8 years, overall outcomes with this cohort were excellent, with 69% and 60% progression-free at 5 and 10 years, respectively, and 96% and 92% alive at 5 and 10 years, respectively.
Among the 12 patients who ultimately sustained a radiographic relapse, peripheral blood became MRD-positive in 11 patients (92%). The first positive MRD test came with a lead time of between 0-24 months (median 70 days) prior to radiographic relapse. Only one patient had a negative MRD result while being found to have progressive disease on imaging. That patient had a single site of disease at the base of tongue which caused symptoms early, despite a very low overall disease burden. Among the 19 patients who have been in continuous clinical remission, 17 have remained MRD-negative. The other 2 had "molecular relapse” (MRD positive without radiographic relapse), which allowed for pre-emptive rituximab treatment, which converted them back to an MRD-negative state. Several patients were able to enjoy 2-4 year intervals without any surveillance imaging, while still getting reassurance every few months from the negative ctDNA test (see Figure 1).
Conclusions Our data suggests that the clonoSEQ® MRD assay is an effective surveillance tool for MCL patients following first-line therapy. In our experience, this MRD assay was predictive of relapse prior to imaging in all but one patient. In addition, the assay allowed for minimization of surveillance imaging, and early detection of MRD allowed for pre-emptive rituximab therapy in select patients.
Disclosures
Hamadani:Gamida Cell: Consultancy; Incyte Corporation: Consultancy; Kite: Consultancy; Takeda: Research Funding; Abbvie: Consultancy; Omeros: Consultancy; ADC Therapeutics: Consultancy, Research Funding, Speakers Bureau; Kadmon: Consultancy; Legend Biotech: Consultancy; Novartis: Consultancy; Medical University of Wisconsin: Current Employment; MorphoSys: Consultancy; SeaGen: Consultancy; Genmab: Consultancy; Spectrum Pharmaceuticals: Research Funding; Astellas Pharma: Research Funding; Sanofi Genzyme: Speakers Bureau; AstraZeneca: Speakers Bureau; BioGene: Speakers Bureau. Shah:Bristol Myers Squibb: Consultancy; Kite Pharma: Consultancy; TG therapeutics: Consultancy; Lilly Oncology: Consultancy, Honoraria; Incyte Corporation: Consultancy, Honoraria, Speakers Bureau; Epizyme: Consultancy; Novartis: Consultancy; Miltenyi Biotec: Consultancy, Research Funding. Fenske:Adaptive Biotechnologies: Consultancy, Speakers Bureau; Beigene: Consultancy; Bristol-Meyers-Squibb: Consultancy, Speakers Bureau; CSL Therapeutics: Consultancy; Karyopharm: Consultancy; Kite (Gilead): Consultancy, Speakers Bureau; MorphoSys: Consultancy, Speakers Bureau; Pharmacyclics (AbbVie): Consultancy; SeaGen: Consultancy, Speakers Bureau; Servier Pharmaceuticals: Consultancy; TG Therapeutics: Consultancy, Speakers Bureau; ADC Therapeutics: Consultancy, Speakers Bureau; Astrazeneca: Speakers Bureau; Sanofi: Speakers Bureau.
Author notes
∗Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal