Abstract
Avatrombopag (AVA) is an oral thrombopoetin (TPO) agonist. In May of 2018 Avatrombopag was FDA approved for use in adults with chronic liver disease (CLD) undergoing a planned procedure. The ADAPT-1 and ADAPT-2 studies compared 40 mg Avatrombopag vs Placebo and 60 mg Avatrombopag vs Placebo. The use of Avatrombopag proved to statistically reduce the need for platelet transfusion, additional procedures to manage post-op bleeding and raised platelet counts to goal of ≥ 50 x 109/L compared to placebo. When using Avatrombopag for a planned procedure the dose is 40 mg for baseline platelet count 40K to 50K and 60 mg for baseline platelet count <40K. Avatrombopag is taken 10 to 13 days before the scheduled procedure which should occur 5-8 days after the last dose.
It is not uncommon for CLD patients to need platelet support from either transfusion or a TPO agonist to achieve a platelet threshold >50K. At our large academic center there are CLD patients who require frequent procedures or need to safely remain on antiplatelet drugs or anticoagulation. This led to offering a role for continuous TPO agonist support with Avatrombopag in select cases.
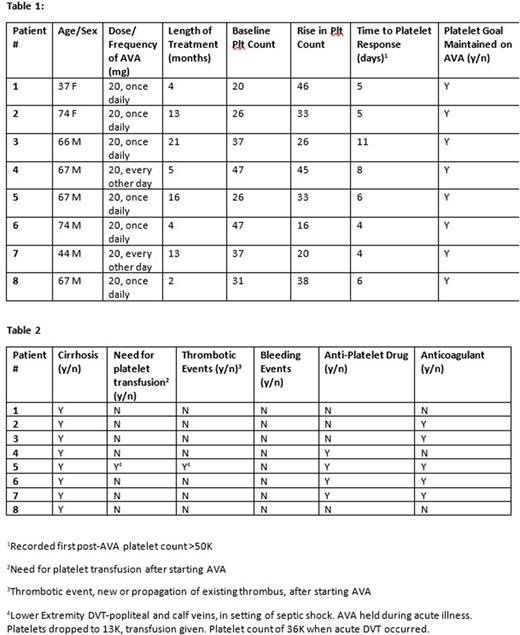
This is a single institute review on the use of Avatrombopag in chronic liver disease. We identified 8 patients with cirrhosis on continuous Avatrombopag between August 2020 and May 2022. The following parameters were gathered on chart review as seen in Table 1 and Table 2: Patient age and sex, dose and frequency of Avatrombopag, length of treatment, baseline platelet count, rise in platelet count, time to platelet response (>50K), maintenance of platelet goal, presence of cirrhosis, need for transfusion after starting Avatrombopag, bleeding events, thrombotic events and use of anti-platelet drug or anticoagulation.
The dose of Avatrombopag needed to maintain a platelet goal >50K was 20 mg daily for 8 out of 10 patients. Two patients only required 20 mg every other day. The median length of treatment was 9 months (range 2-21 months). A median for 5.5 days were required to achieve the platelet goal >50K. All of the patients maintained the platelet goal while taking Avatrombopag. All patients had cirrhosis. Only one patient had AVA temporarily held due to critical illness with no home supply of drug available. During this time platelet count dropped below goal and at this time the patient developed a lower extremity DVT and required platelet support to remain safely on anticoagulation. There were no bleeding events. One patient was on anti-platelet only, two patients were on anticoagulation only and three patients were on both anti-platelet and anticoagulation.
The concern for thrombosis with TPO agonists in cirrhosis has limited trials due to safety concerns. There are limited options for CLD patients requiring frequent procedures, anti-platelet drugs or anticoagulation. The continuous use of Avatrombopag to maintain a platelet goal >50K appears both safe and effective, but does require close monitoring. It is likely that a lower dose of Avatrombopag, 20 mg, can achieve prolonged platelet support. This lower dose could play a role in reducing potential thrombotic risk. In the future a multi-center trial should be pursued.
Disclosures
No relevant conflicts of interest to declare.
OffLabel Disclosure:
Continuous use of Avatrombopag in chronic liver disease. Current FDA approval for short course prior to planned procedure.
Author notes
*Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal