Abstract
Introduction: Arterial thromboembolism is a rising concern in patients with cancer. Data considering incidence and risk factors are scarce. Here we describe arterial thrombosis in a cohort of cancer patients receiving apixaban as treatment for venous thromboembolism.
Methods: A total of 298 cancer patients with any type of venous thromboembolism were treated up to 6 months with apixaban 5 mg twice daily. After six months, the 196 patients left in the study further received secondary prophylaxis with apixaban 2.5 mg twice daily for up to 30 months. Primary efficacy outcome was recurrent venous thromboembolism and safety outcome was bleeding. Arterial thromboembolism was registered as a serious adverse event during the whole study period. This is a post-hoc analysis of the arterial events.
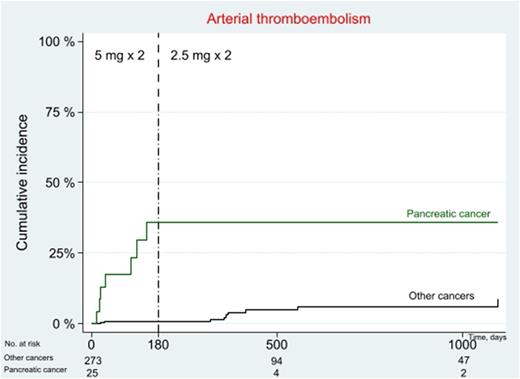
Results: Arterial events were registered in 16/298 (5.4 %, CI: 3.1-8.6) patients. Leucocyte count at baseline were higher (p=0.006) in patients with arterial thromboembolism compared to patients without arterial thromboembolism. Factors that increased the risk of arterial thromboembolism in our patient cohort was pancreatic cancer (OR: 11.41 (95 % CI: 3.81-34.17)), ovarian cancer (OR: 20 (95 % CI: 2.62-152.61)), BMI <22.3 (OR: 3.14 (95 % CI: 1.12-8.79)) and previous venous thromboembolism (OR: 4.673 (1.503-14.524)). Patients with pancreatic cancer had a much higher frequency of arterial thromboembolism than other cancers (figure 1). During the first 6 months, 7/25 (28 %, CI: 12-49)) patients with pancreatic cancer had a stroke. Among pancreatic cancer patients, d-dimer at baseline was higher in those who experienced arterial thromboembolism compared to those who did not (p=0.037).
Conclusion: In our cohort of cancer patients treated for venous thromboembolism with apixaban, there seems to be an increased risk of arterial thromboembolism in patients with pancreatic cancer, low BMI and previous venous thromboembolism. High leukocyte count may be a biomarker for increased risk of arterial thrombosis.
Disclosures
Larsen:Pfizer: Honoraria; Bristol Myers Squibb: Honoraria. Frøen:BMS, Amgen: Consultancy, Honoraria. Porojnicu:BMS: Honoraria. Dahm:Pfizer, Novartis, BMS: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal