Abstract
Introduction
Copy number alterations (CNAs) of chromosome 9p24.1 are frequently observed in patients (pts) with classic Hodgkin lymphoma (cHL), which result in increased expression of programmed death ligand-1 on Reed-Sternberg (RS) cells. The amplification of 9p24.1 was found in approximately 50% of pts with advanced-stage cHL, and was an independent prognostic factor for worse progression-free survival (PFS) (Roemer MG, et al. JCO. 2016). In addition, 9p24.1 CNAs is thought to be one of the potential predictive markers for response of immune checkpoint blockade for cHL (Gerhard-Hartmann E, et al. BJH. 2022). However, the incidence or prognostic impact of 9p24.1 CNAs in elderly pts with newly diagnosed advanced-stage cHL remains unclear.
In this ancillary analysis of a multi-center retrospective study (HORIZON study, UMIN000033264), we evaluated clinical impact of 9p24.1 CNAs in elderly pts with newly diagnosed advanced-stage cHL who received a doxorubicin, bleomycin, vinblastine, and dacarbazine (ABVD).
Methods
The key eligibility criteria of the current analysis were as follows: 1) pts with histologically diagnosed cHL between 2007 and 2016 in each institution; 2) advanced-stage cHL (stage III, IV or IIB with bulky or extranodal lesion); 3) age at diagnosis > 60 years; 4) had received at least one cycle of ABVD as initial treatment including a modified ABVD regimen (dacarbazine dose reduced to 250 mg/m2); and 5) had available specimen for tissue fluorescence in situ hybridization (FISH) analysis. All specimens were centrally reviewed by 3 experienced hematopathologists and confirmed the diagnosis of cHL.
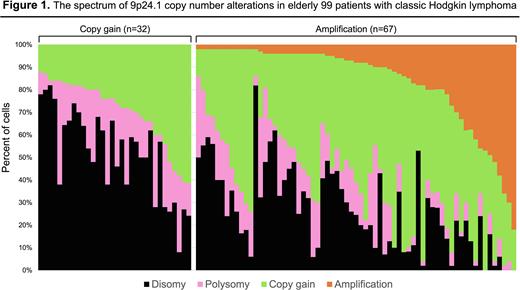
In the tissue FISH analysis, CNAs of the 9q24.1 loci along with the expression of CD30 were simultaneously detected using a combination of immunofluorescence with Fluorescence Immunophenotyping and interphase Cytogenetics as a Tool for Investigation of Neoplasia (FICTION), as described previously (Martínez-Ramírez A, et al. Leukemia. 2004). A median number of 50 RS cells (range: 30 to 74 RS cells) per case were analyzed. According to the previous report (Roemer MG, et al. JCO. 2016), nuclei with a target:control probe ratio of ≥ 3:1 were defined as amplification, and those with a probe ratio of > 1:1 but , < 3:1 were classified as relative copy gain. Nuclei with a probe ratio of 1:1 but more than two copies of each probe were defined as polysomic for chromosome 9p. In each case, the percent and magnitude of 9p24.1 amplification, copy gain, polysomy, and normal copy numbers (disomy) were noted. Cases were classified by the highest observed level of 9p24.1 CNAs.
As survival analysis, overall survival (OS), PFS, and event-free survival (EFS; defined as the time from a diagnosis of cHL to disease progression or relapse, subsequent systemic chemotherapy for cHL, or death due to any cause), were evaluated.
Results
Among 99 eligible pts, the median age was 71 years (interquartile range [IQR] 65-77). The numbers of pts with an ECOG performance status (PS) 2 or more and B symptoms were 17 (17%) and 50 (51%), respectively. Sixty pts (61%) had mixed cellularity cHL, and 28 pts (28%) had nodular sclerosis cHL. Forty-five pts (45%) were positive for EBV-encoded small RNA (EBER) in situ hybridization. Hypoalbuminemia [serum albumin <4 g/dL] and anemia [hemoglobin <10.5 g/dL], were observed in 84 (85%), and 37 pts (37%), respectively.
All the 99 pts had 9p24.1 CNAs; 67 pts (68%) had amplification, and the remaining 32 pts (32%) had copy gain (Figure 1). Hypoalbuminemia and anemia were significantly more frequent among pts with 9p24.1 amplification. The incidence of 9p24.1 amplification in EBER-positive cases tend to be higher than that of EBER-negative cases (78% in pts with EBER- positive vs. 59% in pts with EBER-negative, P=0.056).
With a median follow-up duration of 52 months, the estimated OS, PFS, and EFS at 5 years in pts with 9p24.1 amplification were 80.1%, 64.2%, 59.9%, respectively. The estimated 5-year OS, PFS, and EFS of pts with 9p24.1 copy gain were 71.2%, 58.4%, 55.7%, respectively. No statistical differences were observed regarding these survival parameters between those pts with amplification and copy gain.
Conclusion
This ancillary analysis showed that hypoalbuminemia and anemia were significantly more frequent among pts with 9p24.1 amplification. Nevertheless, 9p24.1 amplification was not a prognostic factor for OS, PFS, and EFS in elderly pts with advanced-stage cHL treated with ABVD in the present study.
Disclosures
Makita:BMS: Consultancy, Honoraria; Chugai: Honoraria; CSL Behring: Honoraria; Daiichi-Sankyo: Consultancy, Honoraria; Eisai: Honoraria; Janssen: Honoraria; Meiji Seika: Honoraria; Novartis: Consultancy, Honoraria; SymBio: Honoraria; Takeda: Consultancy, Honoraria. Kusumoto:Abbvie: Honoraria, Research Funding; Amgen BioPharma: Research Funding; Bristol Myers Squibb: Honoraria, Research Funding; Celgene: Research Funding; Daiichi Sankyo: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; One Pharmaceutical: Honoraria, Patents & Royalties; Pfizer: Honoraria, Research Funding; Sanofi: Research Funding; Takeda: Honoraria, Research Funding. Tamaru:Nichirei Bioscience INC: Research Funding; BMS: Honoraria, Speakers Bureau; Takeda: Honoraria, Research Funding, Speakers Bureau; Chugai: Honoraria, Speakers Bureau; Ridgelinez Limited: Research Funding. Tsujimura:Nippon Shinyaku: Honoraria; Kyowa Kirin: Honoraria; Takeda: Honoraria; Chugai: Honoraria; Eisai: Honoraria; Janssen: Honoraria. Takayama:Chugai Pharmaceutical Co., Ltd.: Honoraria, Research Funding; Takada Pharmaceutical Co., Ltd.: Honoraria, Research Funding; Kyowa Kirin Co., Ltd.: Honoraria, Research Funding; Asahi Kasei Pharma: Honoraria, Research Funding; Pfizer: Honoraria; Janssen Pharmaceutical K.K.: Honoraria; Bristol Myers Squibb: Honoraria. Shimada:Novartis: Consultancy, Honoraria; Meiiji Seika: Consultancy; Celgene: Honoraria, Research Funding; Kyowa Kirrin: Research Funding; Otsuka: Research Funding; Eisai: Honoraria, Research Funding; AstraZeneca: Honoraria; Janssen: Honoraria; Takeda: Honoraria; Symbio: Honoraria; Asclepia: Honoraria; AbbVie: Consultancy; Celgene: Honoraria, Research Funding; Bristol-Meyers Squibb: Consultancy, Honoraria; Kyowa Kirin: Research Funding; Daiichi sankyo: Consultancy, Honoraria, Research Funding; CHUGAI PHARMACEUTICAL: Consultancy, Honoraria, Research Funding. Takahashi:Takeda: Speakers Bureau; Ono: Speakers Bureau; Janssen: Speakers Bureau; Chugai/Roche: Speakers Bureau; Kyowa Kirin: Speakers Bureau; Nippon Shinyaku: Speakers Bureau; Eisai: Speakers Bureau; Mundi pharma: Speakers Bureau; SynBio: Speakers Bureau; AstraZeneca: Speakers Bureau; Meiji Seika: Speakers Bureau. Suzuki:Meiji Seika: Honoraria; Abbvie: Honoraria; Jansen: Honoraria; Celgene: Honoraria; MSD: Honoraria; Bristol Meyer Squib: Honoraria; Eisai: Honoraria, Research Funding; Shionogi: Research Funding; Takeda: Honoraria, Research Funding; Ohtsuka: Honoraria, Research Funding; Taiho: Research Funding; Chugai: Honoraria, Research Funding; Kyowa-Kirin: Honoraria, Research Funding; Sumitomo Dainippon: Honoraria; Novartis: Honoraria; AstraZeneca: Honoraria; Nippon Shinyaku: Honoraria. Tatetsu:Takeda: Honoraria; Novartis: Honoraria; BMS: Honoraria; Chugai: Honoraria; Eisai: Honoraria; Ono: Honoraria; SymBio: Honoraria; Mesoblsat: Patents & Royalties: The applicants are the Brigham and Women's Hospital, INC., Dana-Farber Cancer Institute, INC., National University of Singapore.. Momose:Chugai: Honoraria; Takeda: Honoraria. Asano:Takeda: Honoraria. Maruyama:CHUGAI PHARMACEUTICAL: Honoraria, Research Funding; Kyowa Kirin: Honoraria, Research Funding. Yamaguchi:MSD: Honoraria; Ono Pharmaceutical: Honoraria; Celgene: Honoraria; Genmab: Honoraria, Research Funding; Eisai: Research Funding; Takeda Pharmaceutical: Honoraria, Research Funding; Kyowa Kirin: Honoraria, Research Funding; AstraZeneca: Research Funding; Nippon Shinyaku: Honoraria, Research Funding; Bristol Meyers Squibb: Honoraria; AbbVie: Honoraria; Janssen: Honoraria; SymBio Pharmaceuticals: Honoraria; Meiji-Seika: Honoraria; Chugai Pharma: Honoraria, Research Funding; Sumitomo Dainippon: Research Funding; Daiichi Sankyo: Research Funding; Otsuka Pharmaceuticals: Honoraria, Research Funding. Nagai:Nippon Shinyaku: Honoraria, Research Funding; Celgene: Honoraria; Sumitomo Pharma: Honoraria; Takeda: Honoraria, Research Funding; Parexel: Research Funding; Ono: Honoraria, Research Funding; AstraZeneca: Honoraria, Research Funding; Eli Lilly and Company: Honoraria; Mudi Pharma: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Novartis: Honoraria; Eisai: Honoraria, Research Funding; Chugai: Honoraria, Research Funding; Kyowa Kirin: Honoraria, Research Funding; Daiichi-Sankyo: Research Funding; Solasia: Research Funding; lncyte: Research Funding; IQVIA: Research Funding; Labcorp: Research Funding; Bristol Myers Squib: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal