Abstract
Background
Marginal zone lymphoma (MZL) is a heterogenous group of lymphomas caused by malignant transformation of marginal zone B-cells, further classified into three subtypes: extra-nodal marginal zone lymphoma (EMZL), splenic marginal zone lymphoma (SMZL) and nodal marginal zone lymphoma (NMZL). In 2013, 5-year relative survival rates were reported at 88.7%, 79.7% and 76.5% for EMZL, SMZL and NMZL, respectively (US SEER database).
Aims and objectives
Evidence regarding incidence, treatment pattern, and associated cost for MZL is lacking. This study aims to evaluate the current evidence for epidemiology, treatment patterns, and economic burden associated with MZL.
Methods
Epidemiology and treatment pattern searches were conducted in Embase and Medline-in-process databases (January 2011-February 2022), by a single reviewer and validated by an independent reviewer; STROBE checklist used for critical appraisal. The economic search also included the Cochrane database (up to February 2022), conducted by two reviewers with a third to resolve discrepancies; Adapted Drummond's checklist used for critical appraisal. Relevant conferences websites were also searched. Reviews focused on adult patients with treatment naïve or relapsed/refractory (r/r) MZL.
Results
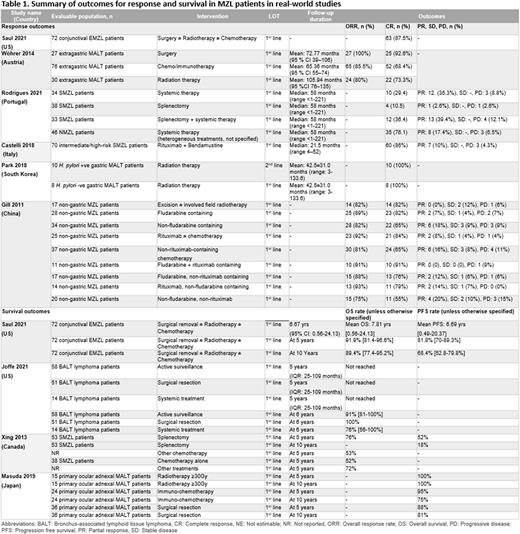
Of the 9089 records retrieved, 91 studies for epidemiology, 32 studies for treatment patterns (RWE: 20, treatment guidelines: 12), and 10 economic studies were included. The studies showed that MZL incidence varied by geographic location (0.5-2.6 per 100,000); and overall incidence of MZL increased over time. The incidence rate was reportedly higher for developing countries vs. western developed countries, older vs. younger patients, and patients of white race vs. other races. Among the MZL population, incidence rate per 100,000 was highest for EMZL/MALT (1.1-1.59), followed by NMZL (0.12-0.83) and SMZL (0.13-0.25). Site-specific incidence per 100,000 was highest for stomach MALT (0.13-0.44). The 5-year relative survival rates for MZL ranged from 61.2-92% and were generally higher in EMZL/MALT patients vs. other subtypes/overall MZL population.
Guideline recommendations for patients with MZL consist of a combination of different therapies including radiotherapy, chemotherapy, immunotherapy and surgery. BTK inhibitors (ibrutinib, zanubrutinib), and rituximab-based combinations are recommended in r/r MZL. Analysis of real-world treatment patterns revealed rituximab±chemotherapy was the most prescribed systemic treatment across all lines of therapy. Common 1L non-systemic treatments included surgical resection (17-100%) and radiation therapy (1.2-86.8%). H. pylori eradication therapy was the most common 1L approach for H. pylori +ve gastric MALT patients. Treatment patterns for patients in subsequent lines were sparsely reported. A large US study (n=1162) reported that MZL patients not receiving an NCCN-recommended agent increased by LOT: 1L (11%), 2L (25%), and 3L (29%); 51% of MZL patients also received no systemic therapy. Studies reporting OS/PFS outcomes (n=4) and response rates (n=6) in real-world patients were limited and predominantly in the 1L setting (Table 1).
Economic studies covered North America and APAC, assessing different measures of economic burden, in MZL sub-populations. No clear trends were observed for costs or resource use by the line of therapy. Overall cost of treatment and AE management was low for BTKi vs. immunotherapy/chemotherapy. Rituximab+bendamustine was shown to be a cost-effective treatment for MZL in Canada, but in the US it incurred greater costs and healthcare resource utilization vs. rituximab monotherapy - potentially indicative of their differing AE profiles.
Conclusion
Whilst MZL incidence varies by geography, incidence rates over time indicate a growing patient population, where the disease and economic burden will continue to increase. The 5-year relative survival rates are highest in EMZL/MALT patient subpopulations, but novel treatments are required across sub-types of MZL. Treatment patterns appear generally reflective of current guidelines, but systemic therapy was heavily reliant on rituximab-based treatment options. Evidence from the US reported that the need for novel therapies increases by LOT, but to further characterize real-world outcomes and treatment pathways in MZL (in particular r/r patients), additional studies are required.
Disclosures
Squires:Merck & Co., Inc.: Current Employment. Saini:Parexel: Current Employment. Pandey:Parexel: Current Employment. Pandey:Parexel: Current Employment. Hall:Parexel: Current Employment. Ryland:Merck & Co., Inc.: Current Employment. Raut:Merck & Co., Inc.: Current Employment. Prescott:Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA: Current Employment, Current holder of stock options in a privately-held company.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal