Abstract
Background: Relapsed or refractory (R/R) primary central nervous system lymphoma (PCNSL) patients and those with isolated CNS relapse of large B cell lymphoma (SCNSL) have poor outcomes with standard treatment. The combination of high dose methotrexate (HD-MTX) and ibrutinib was evaluated in a phase Ib trial in R/R PCNSL (Grommes et al. 2019). There were no dose limiting toxicities (DLTs) or grade 5 adverse events. Overall response rate (ORR) was 80%, with 53% of patients achieving a complete response (CR). The median PFS was 9.2 months.
Pemetrexed has anti-folate activity, similar to methotrexate, but with the advantage of targeting more than one site in the folate metabolism (Adjei et al. 2004). Pemetrexed is active in R/R PCNSL and SCNSL. Pemetrexed was evaluated in 11 patients with R/R PCNSL. Fatigue and hematological toxicities were the most common adverse events (Raizer et al. 2012). In SCNSL, ORR to pemetrexed was 58% in a retrospective study (Zhan et al. 2013). HD-MTX administration requires inpatient care for supportive treatment and toxicity monitoring, while pemetrexed is administered on outpatient basis. Zanubrutinib is a BTK inhibitor with a favorable side effect profile and an excellent blood brain barrier penetration. Several small trials reported zanubrutinib activity in CNS lymphoma (Zhang et al. 2021, Cheng et al. 2021). We hypothesize that pemetrexed in combination with zanubrutinib (Pem-Zan) is safe and will produce higher ORR than pemetrexed alone in patients with R/R PCNSL or SCNSL.
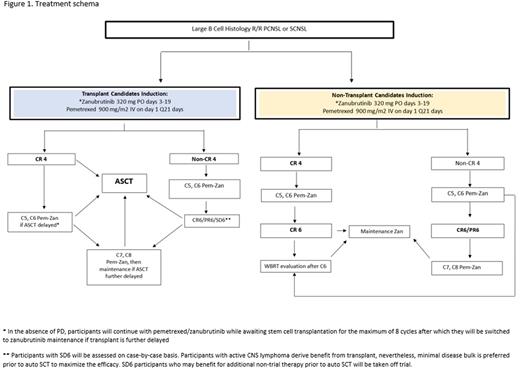
Design and methods: This is a single center phase II trial with a safety lead-in portion to evaluate the safety and activity of Pem-Zan combination as induction therapy in patients with R/R PCNSL and SCNSL. Eligible patients will have confirmed large B cell histology R/R PCNSL or SCNSL, KPS ≥ 30% (≥ 50% for age ≥ 60 years) and good organ function. Patients with HIV will be included if they have negative viral load and CD4 count >400, non-viremic hepatitis C and B positive will be included. Induction therapy consists of pemetrexed 900 mg/m2 IV every 3 weeks and zanubrutinib 320 mg P.O. daily on days 3 to 19 of each 21-day cycle. Zanubrutinib is scheduled to avoid concomitant use with pemetrexed due to reports of antagonistic relationship between BTK inhibitors and anti-folate agents (Lionakis et al. 2017). Zanubrutinib maintenance dose is 320 mg P.O. daily. The safety lead-in will enroll 6 patients. Accrual will be haltered if excessive number of DLTs is seen according to a Pocock-type stopping boundary. The Simon's 2-stage MiniMax design will be employed for the second part of the study.
Transplant eligible patients will proceed to autologous stem cell transplant (ASCT) once CR is achieved, or after up to 8 cycles of induction if in PR. Non-ASCT candidates will receive Zan maintenance after the maximum of 8 cycles of Pem-Zan induction. All non-ASCT candidates in CR after cycle 6 of induction will be offered consolidative whole brain radiation therapy (WBRT) followed by Zan maintenance (Figure 1).
The primary endpoint is the best ORR to Pem-Zan induction therapy using International PCNSL Collaborative Group's criteria (Abrey et al. 2005). The secondary endpoints include ORR after transplant, WBRT followed by zanubrutinib maintenance or zanubrutinib maintenance; CR rate post-induction, CR rate post-transplant; clinical benefit (CR, PR, SD) rate, duration of response, progression free survival, overall survival, and type/frequency of DLTs. We will correlate cell of origin by Hans algorithm (Hans et al. 2004), chromosomal abnormalities, and tumor mutational profiles with clinical outcomes. We will study the significance of circulating tumor DNA presence and quantity in blood and CSF by next-generation sequencing combined with multiplex PCR using clonoSEQ assay technology (Olszewski et al. 2021).
Status: the study is currently pending submission to FDA
Funding: BeiGene Inc., Baptist Health Foundation
Disclosures
Linhares:Genentech: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol Myers Squibb: Other: advisory board, Research Funding; ADC therapeutics: Other: advisory board, Research Funding; Gilead Sciences, Inc.: Other: advisory board; glaxosmithkline: Other: advisory board; Seagen Inc: Other: advisory board, Research Funding; BeiGene USA, Inc.: Other: advisory board, Research Funding; Alexion: Membership on an entity's Board of Directors or advisory committees; Abbvie: Other: advisory board; TG therapeutics: Other: advisory board; Kyowa Kirin: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AstraZeneca: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Curio Science: Other: Workshop Participation and Moderation. Nahas:BMS: Speakers Bureau; Janssen: Speakers Bureau; GSK: Honoraria; Blueprint: Speakers Bureau; Gilead: Honoraria; GSK: Honoraria; BLUEPRINT: Speakers Bureau. Feinstein:BMS: Consultancy, Speakers Bureau; Amgen: Consultancy, Speakers Bureau. Odia:Bristol myers: Consultancy, Research Funding; Gamma tile: Consultancy, Other: Advisory or consulting role.
OffLabel Disclosure:
In this phase II study with a safety lead-in, we combine pemetrexed and zanubrutinib for the treatment of relapsed and refractory primary and secondary CNS lymphoma. There are no chemotherapy agents specifically approved for primary or secondary CNS lymphoma (PCNSL or SCNSL). For example, methotrexate is a backbone for primary and secondary CNS lymphoma regimens, but has no specific FDA approval for these indications. Likewise, other chemotherapeutics and targeted agents used for PCNSL or SCNSL, such as cytarabine, temozolomide, rituximab, ibrutinib, lenalidomide do not have FDA approvals for these indications. Pemetrexed has anti-folate activity, similar to methotrexate, but with the advantage of targeting more than one site in the folate metabolism (Alimta PI, 2004). Pemetrexed is FDA approved for the treatment of mesothelioma and locally advanced or metastatic nonsquamous non-small cell lung cancer and has demonstrated sufficient activity in relapsed and refractory PCNSL and SCNSL to be included in NCCN guidelines for the treatment of relapsed and refractory PCNSL (Alimta PI 2019, NCCN 2022). Pemetrexed is administered in the outpatient setting and as opposed to high dose methotrexate does not require complex care such as hydration or antidote administration. Pemetrexed has an excellent toxicity profile with most frequent toxicities being fatigue and cytopenias (Dietrich, 2020). Zanubrutinib is a kinase inhibitor FDA approved for the treatment of adult patients with mantle cell lymphoma (MCL) who have received at least one prior therapy, Waldenströmââ,¬â"¢s macroglobulinemia, relapsed or refractory marginal zone lymphoma (MZL) who have received at least one anti-CD20-based regimen (Brukinsa PI, 2021). Zanubrutinib has excellent blood brain barrier penetration (Zhang, 2021). Zanubrutinib demonstrated activity in CNS lymphoma in multiple small studies and case reports (Zhang, 2021, Yang, 2021, Wong, Hemasphere 2018, Wang, 2021, Cheng, 2021). Ibrutinib and zanubrutinib share a similar mechanism of action, while zanubrutinib being more selective, exhibits less off-target inhibition than ibrutinib and has a potential for less off-target toxicity than first-generation BTK inhibitors. Zanubrutinib may be better tolerated in combination treatment than ibrutinib due to known lower frequency of side effects such as diarrhea, headaches, and hypertension (Sawalha, 2020).
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal