Abstract
Introduction
B-cell non-Hodgkin lymphoma (NHL) is the most common hematological malignancy encompassing heterogenous subtypes (Dotan et al., PT 2010). Rituximab (R) has changed the treatment landscape of NHL; approvals of rituximab biosimilars have improved access to and the affordability of this treatment (Mohammed et al., J Blood Med 2019).
HLX01 is the first rituximab biosimilar approved for the treatment of NHL and chronic lymphocytic leukemia in China. In a Phase 3 trial enrolling patients with diffuse large B-cell lymphoma (DLBCL), HLX01 demonstrated bioequivalence to reference rituximab in terms of efficacy and safety (Shi et al., J Hematol Oncol 2020).
The current study is an ongoing, observational study with the objectives of characterizing the distribution of B-cell NHL subtypes in patients receiving HLX01 and assessing the effectiveness and safety of HLX01 in patients with B-cell NHL subtypes in a real-world setting in China.
Methods
This multicenter, real-world study enrolled adult patients with pathologically confirmed B-cell NHL subtypes at 40 hospitals in China. HLX01 (either as monotherapy or combination therapy) was recommended, but the selection of treatment regimen was at the investigator's discretion. The primary endpoint was the distribution of B-cell NHL subtypes in patients receiving HLX01. Secondary endpoints were objective response rate (ORR) and complete response (CR) rate in patients with B-cell NHL and progression-free survival (PFS) and overall survival (OS) rates at 1, 3, and 5 years in subgroups categorized by disease characteristics. Response was evaluated every 6 weeks until disease progression or end of treatment, using the Lugano 2014 criteria. After treatment, disease and survival status were followed up every 2 months. Safety was monitored until 1 month after treatment completion. Here, we report results from baseline to 1-year follow-up.
Results
Between April 2020 and June 2021, 2053 patients were enrolled; of these patients, 1898 had evaluable efficacy data (full analysis set [FAS]) and 1878 had completed ≥ 5 treatment cycles (per-protocol set [PPS]).
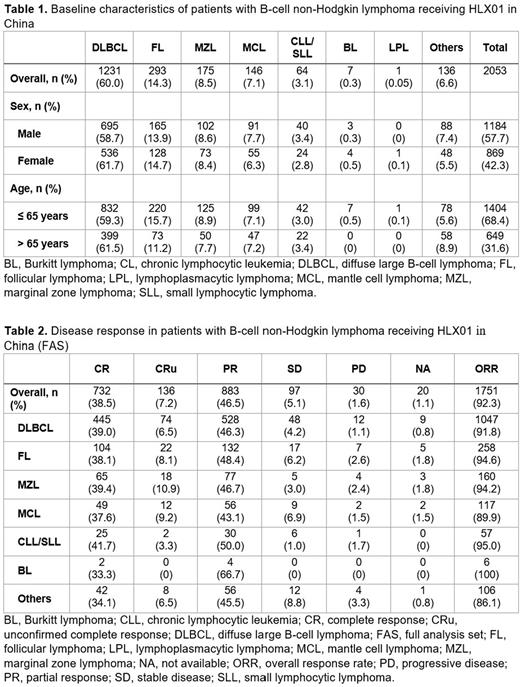
Overall, the most common subtype was DLBCL (1231/2053, 60.0%), followed by follicular lymphoma (293/2053, 14.3% [Table 1]). Among patients in the FAS, R-CHOP was the most commonly used regimen (1006/1898, 53.0%). Other regimens included BR (175/1898, 9.2%), R monotherapy (150/1898, 7.9%), R2 (63/1898, 3.3%), R-EPOCH (56/1898, 2.95%), DA-R-EPOCH (48/1898, 2.5%), R-DA-EDOCH (18/1898, 0.95%), R-EDOCH (11/1898, 0.58%), and others (371/1898, 19.5%).
In the FAS, 868 (45.7%) patients had CR/CRu (CR + unconfirmed CR) and 883 (46.5%) had partial response, contributing to an ORR of 92.3%; in the PPS, the ORR was 93.2%. In 215 patients with evaluable OS and PFS data, median OS and PFS were not reached, and 1-year OS and PFS rates were 90.7% and 74.4%, respectively.
In patients with DLBCL in the FAS, 519 (45.5%) had CR/CRu and the ORR was 91.8%. In 139 evaluable patients with DLBCL, 1-year OS and PFS rates were 91.3% and 75.6%, respectively. The ORRs in most subtypes exceeded 90% (Table 2).
Among all patients, 423 (20.6%) experienced adverse events (AEs), most of which were grade 1 or 2 in severity. The most common AEs were decreased white blood cell count (3.9%), decreased neutrophil count (3.5%), and nausea/vomiting (3.3%).
Conclusions
DLBCL is the most common subtype in HLX01-treated patients with B-cell NHL in China. HLX01 showed clinical benefits with favorable safety profiles in patients with B-cell NHL subtypes in a real-world setting.
Clinical trial identification: ChiCTR2000032263. Research funding: None.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal