Abstract
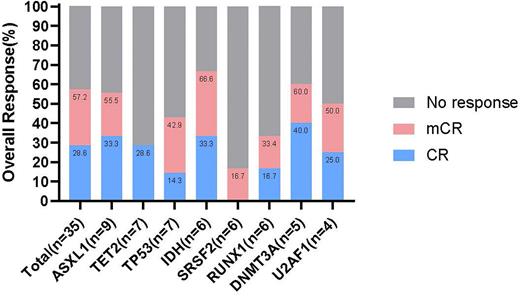
The treatment of patients with refractory and/or relapsed (R/R) high-risk myelodysplastic syndrome (HR-MDS) remains a daunting clinical challenge. Venetoclax is a selective BCL-2 inhibitor, which combined with hypomethylating agents (HMAs), increased responses and prolonged survival in unfit and previously untreated AML. We performed a retrospective study of patients with R/R HR-MDS receiving combination azacytidine (AZA) plus 15-days duration of Venetoclax (VEN-15d) in order to determine their efficacy and toxicity in this context. We showed that the overall response rate (ORR) was 57.2% (20/35) and the median over survival (OS) was 14 months in R/R MDS. The most common treatment-emergent adverse events were peripheral blood cytopenias and infectious complications. Our retrospective study showed that the real-world experience of treating R/R MDS with AZA plus VEN-15d highlights an encouraging response rate with myelosuppression being the major toxicity. Of note, VEN-15d with AZA may salvage patients failing to respond optimally to HMAs and reduce the disease-burden for subsequent allo-SCT in our analysis. These data of combination AZA plus VEN-15d in R/R MDS warrant further prospective evaluation in clinical trials.
Disclosures
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal